
Current techniques of endoscopic decompression in spine surgery
Introduction
Minimally invasive spine surgery (MISS) has become a mainstream concept in spine medicine. Back or neck pain with radiculopathy is one of the common reasons for patients to opt for extensive treatment, and conventional surgical procedures for spinal disc disease or stenosis include open decompression with or without fusion surgery. However, perioperative complications and prolonged recovery time after surgery have emphasized the need of MISS (1,2). The goal is to minimize normal tissue trauma during the surgical approach while providing the same therapeutic effect. As a result of MISS, the patient can return to normal life earlier with less adverse impact and maintain a high quality of life.
Endoscopic spine surgery, in this viewpoint, can utilize the essential concept of MISS. Use of endoscopic technology in spine surgery can offer a minimally invasive, percutaneous approach rather than the wide-open surgical exposure (3,4). It may also provide an excellent and selective visualization of the lesion. Until now, most endoscopic spine surgeries have been developed for endoscopic discectomy or decompression techniques.
This review article aimed to describe the current endoscopic decompression techniques and to discuss the benefits, limitations, and future perspectives of this minimally invasive technique.
Classification and terminology of the endoscopic decompression techniques
Endoscopic spine surgeries vary based on the disease entities, spinal levels, approach, and endoscopic properties used (Table 1).

Full table
In terms of spinal disease for endoscopic spine surgery, there are two major disease entities: disc herniation and stenosis. Any thecal sac or nerve root compression due to herniated disc or spinal stenosis can be the primary candidate for endoscopic decompression techniques. Generally, endoscopic decompression techniques are not suitable for other spinal conditions, such as segmental instability, tumor, trauma, infection, and deformity. The most basic level for endoscopic decompression is the lumbar or lumbosacral level, followed by the cervical and thoracic levels. The approach can be variable based on the purpose of the surgery: transforaminal, interlaminar, anterior, posterior, caudal and other approaches. The last and most critical factor for categorization is the used endoscopic property: percutaneous endoscope, microendoscope, biportal endoscope, and epiduroscope.
According to the property of the endoscope used
Percutaneous endoscopic (full-endoscopic) system
The most commonly used system in endoscopic spine surgery is the percutaneous endoscopic or full-endoscopic system. This is typically characterized by the following (5,6): (I) use of a working channel endoscope with the working channel and the optics in the same tubular device; (II) complete percutaneous approach with a stab skin incision, and (III) utilization of a monoportal approach with continuous saline irrigation. This technique was developed in the mid-1980s and has become the standard endoscopic spine surgery (3,4,7,8) (Figure 1A).

Microendoscopic system
The second most frequently used endoscopic device is the microendoscopic system. This category involves using a rigid endoscope (microendoscope) attached to a tubular retractor with tissue dilators, which help minimize muscle retraction (9-12). The most commonly used system is the METRx tube assembly (Figure 1B). However, unlike the other endoscopic systems, this system is not a water-based procedure, and constant saline irrigation is not used. In real practice, it is frequently applied as a minimally invasive microscopic surgery with a tubular retractor system instead of an endoscopic assembly.
Biportal endoscopic system
The third category of the endoscopic spine surgery is characterized by the use of an endoscopic or arthroscopic system with separate optical and working channels (13-16). This concept is similar to arthroscopic joint surgery, wherein two working portals are needed: the endoscopic portal and the instrumental portal. The endoscopic portal is used for viewing of the surgical field with constant saline irrigation, whereas the instrumental portal is used for surgical instrumentation and procedure (Figure 1C).
According to the method of approach
Transforaminal (posterolateral) approach for lumbar/thoracic spine
The transforaminal approach refers to a posterolateral percutaneous approach to the disc or epidural space through the foraminal window while preserving the normal musculoskeletal structures. The most critical benefit of this approach is that it may provide direct access to the pathologic point without requiring a large skin incision, wide muscle retraction, unnecessary bone resection, and general anesthesia (Figure 2A).
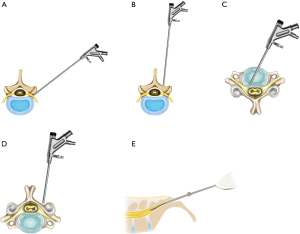
Interlaminar (posterior) approach for the lumbar/thoracic spine
This technique is characterized by a percutaneous posterior or interlaminar approach to the epidural space or disc pathology. The interlaminar approach is familiar to general spine surgeons, because it is similar to that of open microscopic lumbar/thoracic decompression. The decompression processes are also similar to those of open microscopic decompression (Figure 2B).
Anterior approach for cervical spine
The standard surgical option for cervical disc disease is anterior cervical discectomy and fusion. Likewise, the anterior percutaneous endoscopic approach may be the standard technique for endoscopic cervical decompression technique. The targeting and disc decompression are relatively easier without thecal sac retraction (Figure 2C).
Posterior approach for cervical spine
In the case of foraminal disc herniation or foraminal stenosis in the cervical spine, the posterior percutaneous endoscopic cervical foraminotomy and disc decompression can be more effective. The percutaneous endoscopic approach is easier, and the bony foraminal decompression is feasible with endoscopic burrs (Figure 2D).
Caudal approach for lumbosacral spine
The caudal approach, or trans-sacral approach, involves insertion of the small fiberoptic endoscope, or epiduroscope, through the sacral hiatus. This concept of the approach is similar to that of cardiac or cranial vascular intervention. However, the surgical space is very narrow, so the used endoscopic system is small and limited. Therefore, the definitive decompression effect is limited to small disc herniation at the current technical status (Figure 2E).
Representative endoscopic decompression techniques
Until now, many kinds of endoscopic decompression techniques have been introduced. Among them, the following methods have been the most commonly studied and applied in real practice throughout the history of endoscopic spine surgery: percutaneous endoscopic lumbar discectomy (PELD), percutaneous endoscopic decompression (PED) for lumbar stenosis, and percutaneous endoscopic cervical discectomy (PECD).
Transforaminal PELD
Transforaminal PELD is the representative endoscopic spine surgery with a long history and full application. The initial indication of this technique was soft lumbar disc herniation in various situations. Given the advancement in endoscopic technology, its practical application has widened, and has included migrated, recurrent, foraminal, extraforaminal, and even partially calcified disc herniations. This technique has been proven by many randomized trials, meta-analyses, and systematic reviews (17-24). The basic concept of this technique is the direct access to the disc pathologies through the intervertebral foramen or safety working zone while preserving the normal tissues. It could be argued that this is the best technique for implementation of MISS.
The patient is placed in prone position on a radiolucent table. The procedure is usually performed under local anesthesia or conscious sedation. A posterolateral transforaminal lumbar approach is performed under fluoroscopic control. The approach needle is inserted into the herniated disc through the foraminal window, avoiding the exiting nerve root. After discography, the needle is replaced serially by the guidewire, dilator, and final working cannula. Then the working channel endoscope is inserted, and a selective discectomy and epidural decompression can be performed. Success can be achieved through the following technical keys: precise discrimination of endoscopic anatomy, the release of annular anchorage, and removal of the whole herniated fragment. First, the anatomical layers, including the herniated disc, annular fissure, posterior longitudinal ligament, and neural tissues, should be discriminated precisely. Second, the herniated disc fragment should be released from the annular anchorage or adhesion using micropunches and annulus cutter. Third, the released hernia fragment should be removed entirely without any loose pieces in the epidural and intradiscal space. Finally, the endpoint of the procedure should be adequately determined by a free mobilization of the neural tissue and strong pulsation of the dural sac.
Transforaminal PED for lumbar lateral recess/foraminal stenosis
The most common pathology of the lumbar lateral recess stenosis and foraminal stenosis is hypertrophy of the superior articular process (SAP). As a result, the traversing nerve root is compressed in the lateral recess stenosis and the exiting nerve root is compressed in the foraminal stenosis. The transforaminal endoscopic approach can be suitable for the treatment of the lateral recess/foraminal stenosis by resection of the hypertrophied SAP.
Unlike PELD, the working cannula is usually docked within the foramen, not in the disc space, in PED. The safety docking zone of the working cannula is the caudal surface of the SAP and pedicle. This is the typical out-to-in technique described by Schubert and Hoogland (25-27). The tip of the SAP may be typically removed by a bone trephine or endoscopic burrs. After sufficient removal of the bony stenosis, the exposed ligamentum flavum can be subsequently removed by micropunches or forceps. For the lateral recess stenosis, the caudal part of the foramen and the traversing nerve root are decompressed, whereas for the foraminal stenosis, the cranial part of the foramen and the exiting nerve root are decompressed. Additional pedicle resection may enhance the decompression effect. The key to success in this technique is the adequate landing of the working cannula and sufficient decompression of the critical point, which is usually located around the hypertrophied SAP and thickened ligamentum flavum. The technical difficulties that interpose with complete decompression are safe engagement of the working cannula within the foramen, extraforaminal or epidural bleeding, and confusion in the endoscopic anatomical discrimination. Therefore, this technique is regarded as more challenging than the standard endoscopic discectomy.
Interlaminar PELD
In fact, the interlaminar PELD was developed for lumbar disc herniation at the L5–S1 level with high iliac crest. In this case, a usual transforaminal approach is difficult or even impossible. Some expert surgeons developed the interlaminar approach to overcome this problem (28,29). They found that the interlaminar space of the L5–S1 level is usually large enough to pass the endoscopy and working cannula. This technique uses a posterior interlaminar approach with the small working cannula in the epidural or intradiscal space, while preserving paraspinal musculatures and lamina. It can also be applicable to the other levels by using endoscopic punches or drills to enlarge the interlaminar space for introduction of the working cannula and instruments. A standard spine surgeon can be more familiar with the interlaminar approach than the transforaminal approach. In fact, the presence of the innocent exiting nerve root during the transforaminal approach can be stressful for the endoscopic spine surgeons. This technique has evolved and eventually become the interlaminar endoscopic lumbar decompression technique for lumbar stenosis.
Interlaminar PED for lumbar central/lateral recess stenosis
As the size of the working channel endoscope and associated instruments became bigger, a definitive endoscopic decompression technique for lumbar central or lateral recess stenosis was developed.
The definitive indications for interlaminar PED are as follows: (I) central or lateral recess stenosis on magnetic resonance imaging (MRI) and computed tomography (CT) scan without a foraminal stenosis; and (II) neurogenic claudication with leg pain with or without motor weakens.
The surgical technique can be performed using the standard method described in previous studies (28,29). The patient is placed in a prone position under general or epidural anesthesia. The initial target point is the lateral edge of the interlaminar window. After serial dilation, the final working cannula was placed on the lamina surface. Endoscopic laminotomy was performed from the medial border of the superior facet using the endoscopic burr and bunches. Decompression can proceed including cranial and caudal laminotomy, medial facetectomy, and removal of the ligamentum flavum. In the case of bilateral decompression, further decompression of the contralateral side is needed after ipsilateral decompression. The endoscope and the working cannula were directed toward the contralateral side, dorsal to the dural sac. At this point, it is better to leave the ligamentum flavum intact to protect the dural sac during the contralateral laminotomy. The undercutting technique over the ligamentum flavum should be performed until the medial aspect of the contralateral facet can be reached. The remaining ligamentum flavum is then completely removed using the endoscopic punches and other supplementary instruments. All surgical fields were manipulated under endoscopic visual control and constant saline irrigation.
Anterior PECD
The main disease entity for PECD is soft cervical disc herniation with or without foraminal stenosis. The following two approaches are used for cervical disc herniation: anterior and posterior. The approach direction can be determined according to the zone of disc pathology. The anterior approach is effective for cervical disc herniation cases in which the main herniation is located medial to the lateral edge of the myelon.
Anterior endoscopic decompression for the cervical level has theoretical benefits compared to the lumbar level (30). First, the cervical nerve root is usually confined to a smaller space. Second, a slight volume reduction leads to a more significant effect. Third, topographically, the approach is performed in an anterior-posterior direction. Thus, targeting is more comfortable and more precise. Any zones of disc herniation can be treated with anterior PECD, including central, paracentral, and foraminal disc herniations. It also has typical advantages of the minimally invasive percutaneous approach. Minimal skin incision and muscle retraction may reduce the risk of hematoma, infection, vocal cord palsy, and injury of significant structures, such as the carotid artery, trachea, and esophagus. The procedure can be performed under local anesthesia. Therefore, it will be useful for the elderly or medically compromised patients. However, there are some limitations associated with this technique. First, the central nucleus may be disrupted by the anterior percutaneous approach, thereby postoperative disc space narrowing or instability may occur. Second, this approach cannot be applied in case of disc space narrowing or advanced spondylosis.
The general indications for anterior PECD were as follows: (I) soft cervical disc herniation at any zone of the cervical disc; (II) unilateral cervical radiculopathy without central stenosis or foraminal stenosis.
The surgical technique was performed using the standard method described in previous studies (31-33). The patient was placed in a supine position, and the procedure was performed under local or general anesthesia. The approach needle was inserted from the contralateral side to the intradiscal space through a safe working zone between the carotid artery and the tracheoesophagus. After a serial dilation process, the final working cannula was placed intradiscally with the tip of the working cannula on the posterior vertebral line. A working channel endoscope was then inserted, and the intradiscal structures were examined. Then, a selective discectomy was performed using endoscopic forceps and supplementary radiofrequency or laser. The anterior and central nucleus should be preserved to avoid postoperative disc collapse, while the herniated fragment at the posterior part of the disc is completely removed.
The overall success rate of the anterior PECD is variable from 51% to 95% (30-33). According to the randomized trial of Ruetten et al. (34), the anterior PECD technique is a sufficient and safe alternative to conventional surgery with benefits of minimally invasive intervention when the indication criteria are fulfilled.
Posterior percutaneous endoscopic cervical foraminotomy and discectomy
The main target for this procedure is foraminal cervical disc herniation or foraminal stenosis. Given that the cervical spinal cord should not be retracted medially, the posterior approach is useful for cervical disc herniation, in which the primary pathology is located lateral to the lateral edge of the myelon. The main indications for posterior PECD are as follows: (I) foraminal/lateral cervical disc herniation; (II) unilateral cervical foraminal stenosis with intractable cervical radiculopathy.
The surgical procedure can be performed according to the standard technique (35). The procedure is performed under general or local anesthesia, with the patient placed in a prone position. The main target point of the approaching needle is the laminofacet junction (so-called “Y-point”). After serial dilation, the final working cannula is placed on the laminofacet junction under fluoroscopic guidance. A working channel endoscope is then introduced and the bony structures were examined. The foraminal unroofing and foraminotomy are then performed using endoscopic burrs around the Y-point. The extent of facet removal is limited to 50% of the facet joint (36). After adequate foraminotomy, a selective endoscopic discectomy is performed. After identification of the exiting nerve root, the extruded disc can be removed using a dissector and forceps. The endpoint of the procedure can be achieved by free pulsation or mobilization of the nerve root from the proximal to the distal exiting zone.
The clinical outcomes of the posterior PECD technique have been reported to be comparable to those of open cervical surgery (35,37). According to the randomized trial of Ruetten et al. (35), the posterior PECD can be an effective alternative to conventional open surgery in adequately selected cases.
Discussion
Principal concepts and clinical importance of endoscopic spine decompression surgery
The most fundamental concept of endoscopic spine surgery is minimal normal tissue trauma during the surgical approach while maintaining its curative effectiveness on the spinal pathologies. By conducting this mission, the surgical morbidities or complications may be reduced, and the recovery time may be minimized. There are several conditions to characterize the endoscopic spine surgery. First, the approach is usually carried out percutaneously with a small tubular retractor or other devices. Second, surgical access is generally performed through some specific windows that can provide a valid surgical field for treating the spinal pathologies. Third, the endoscopic visualization can provide a wider visual field compared to that of microscopic visualization. Finally, many of the procedures can be frequently performed under local or regional anesthesia, especially for the elderly or medically compromised patients.
Pros and cons of endoscopic spine decompression surgery
The main objective of endoscopic spine surgery is to reduce iatrogenic tissue trauma and to maintain proper segmental stability and mobility. The definitive benefits of endoscopic spine surgery over conventional open surgery may be summarized in three ways. First, the minimized tissue damages such as small skin incision, inessential need for extensive lamina/facet resection or dural sac retraction, and reduced blood loss are apparent. Second, outpatient surgery or same-day surgery is feasible, owing to local anesthesia combined with conscious sedation, reduced operative time, and shorter length of hospital stay. Finally, an earlier recovery may be obtained due to less postoperative medication, fewer wound complications, and quicker return to regular work (5,17,18,20). Therefore, the endoscopic spine surgery may be a practical alternative for elderly or medically compromised patients who are at risk for extensive open surgery under general anesthesia. However, there are also limitations or disadvantages related to endoscopic spine surgery. First, some endoscopic surgery-specific complications can occur and should be addressed to establish the relevance of spinal endoscopy. The rate of common perioperative complications such as epidural hematoma, dorsal dural tear, and surgical site infection can be relatively low. However, there may be some unique adverse events such as a ventral dural tear, nerve root injury to exiting nerve root or innocent neural tissue, and increased radiation exposure (38-43). Second, the learning curve is relatively long and challenging before ensuring clinical success without complications (44-47). The surgeon’s skill or proficiency may influence surgical outcomes. Most surgeons or practitioners do not have opportunities to learn the endoscopic spine surgery during residency or fellowship, however systematic training in endoscopic techniques and anatomical knowledge are required before independently performing the technique in a real situation. Finally, feasible indications are still limited. A calcified disc, severe stenosis, cauda equina syndrome, painless weakness, or severe fibrotic tissue adhesion may be the contraindications for endoscopic surgery. Therefore, appropriate patient selection is an essential key to success. Most patients do not want extensive open surgery or general anesthesia. Thus, they want to undergo percutaneous or minimally invasive “procedures” under “local anesthesia.” Given the needs of patients, the surgeon or practitioner are apt to apply the endoscopic spine surgery beyond the actual indication. This tendency may cause considerable incomplete decompressions or complications because of the “overuse” of the procedure. Thus, patient selection of the endoscopic spine surgery technique should be strictly applied.
Patient selection of endoscopic spine decompression surgery
The essential keys to the clinical success of endoscopic spine surgery are adequate patient selection and suitable operative technique. These two conditions can be obtained from a systematic training course and the surgeon’s experience. Nowadays, various opportunities to learn how to achieve proficiency in the endoscopic surgical technique have been provided by systematic training courses, including hands-on or cadaver workshops. However, the importance of proper patient selection or surgical indications has been underestimated. The most common causes of failed endoscopic spine surgery are either intraoperative adverse events or incomplete decompression (48,49). Careful evaluation of preoperative neurological status and imaging studies may predict some surgical failure. In the case of painless, profound motor weakness, the endoscopic approach is usually contraindicated. In the case of a conjoined nerve root in the neural foramen, a transforaminal endoscopic procedure should be avoided, because the exiting nerve root can be damaged during the transforaminal approach. The practitioner can be informed not only of the type of disc herniation or stenosis, but also of the feasibility of the endoscopic access by checking imaging studies such as simple X-rays, CT scans, and MRIs. The zone of disc herniation or stenosis, degree of canal compromise, severity of adhesion, risk of dural tear, softness of the disc, and degenerative change should be evaluated. For proper patient selection and clinical success of endoscopic decompression, the surgeon should keep in mind the technical limitations compared with the patient’s demographical and radiological conditions.
Technical considerations
Another key to the success of endoscopic spine surgery is precise surgical technique; the practitioner should be highly skilled at the given endoscopic surgery. We can encounter multifarious conditions in real practice at L5–S1 or C6–7 or other inaccessible levels such as migrated disc herniation, highly compromised spinal canal, stenotic foramen, advanced spondylosis. For those cases, a specialized technique may be required for effective decompression to avoid complications.
Regarding the transforaminal approach, the landing point should be as close as possible to the target pathology and as far as possible from the exiting nerve root. Then, the anatomical layers should be discriminated during the decompression procedure. Finally, any herniated disc or soft tissues should be removed entirely after the adequate releasing process. The endpoint of the procedure can be determined by free mobilization of the nerve root and a steady pulsation of the dural sac.
In the interlaminar approach, the essential concern is a dural tear. To prevent a dural breach, the practitioner should differentiate the dural sac, traversing nerve root, and exiting nerve root during the tissue dissection. Any tissue adhesion or anchorage should be dissected and released during the discectomy or laminotomy procedure. In the case of intraoperative bleeding and blurred vision, complete hemostasis should be confirmed before instrumental decompression. Adequate hemostasis can be achieved by the combination of bipolar coagulator, high-water pressure, and hemostatic agents.
In the anterior cervical approach, the essential technical factor is the precise targeting of the disc pathology. The surgeon should feel the carotid pulse and push down the anterior neck into the space between the carotid artery and tracheoesophagus until the fingertips touch the anterior surface of the vertebral body (30). The tracheal air shadow on the fluoroscopic view may be a good indicator for the position of tracheoesophagus. For the patient with a short and thick neck, the shoulder shadow may interfere with the C6–7 or lower level. An oblique fluoroscopic view can be useful to approach the C6–7 level. Regarding the selective discectomy, direct fragment removal with small instruments is difficult because of tenacious annular anchorage. A careful release of fibrotic adhesion around the herniated fragment is mandatory before removal of the freely movable herniation fragment.
In the posterior cervical approach, a definitive dissection of bony structures and identification of the laminofacet junction (so-called “Y-point”) is essential for a safe and precise cervical foraminotomy. The extent of facetectomy should be limited to no more than 50% of the facet joint to prevent postoperative instability. After adequate foraminotomy, the herniated foraminal disc fragment should be removed while preventing a dural tear. The dissection between the herniated disc and the neural tissues can be performed with a blunt dissector. The exposure of herniated fragment with firm nerve retraction can be achieved by rotating the bevel ended tip of the working cannula. After adequate nerve retraction, the herniated piece can be removed by endoscopic forceps and supplementary radiofrequency or laser. Epidural bleeding may occur from flourishing venous plexus. A gentle tamponade with hemostatic agents or hydrostatic pressure may be useful with a bipolar coagulator.
Scientific evidence
A different kind of endoscopic spine surgery technique has been emerging since Hijikata, Kambin and Sampson developed the posterolateral percutaneous lumbar discectomy in the mid-1970s. The levels of evidence are variable according to the history of each endoscopic procedure. Up to date, only transforaminal endoscopic lumbar discectomy technique has been proven by some randomized trials (17-20). According to the updated systematic reviews and meta-analysis, the therapeutic effects of the transforaminal endoscopic lumbar discectomy is not inferior to those of standard open lumbar microdiscectomy in terms of the success rate, complication rate, and recovery time (22-24). The second ranked endoscopic spine surgery technique is the interlaminar endoscopic lumbar discectomy. Some published randomized trials and a few meta-analyses prove that this endoscopic technique is also not inferior to the standard open surgery (50-53). In the cervical spine, some authors have published randomized trials of anterior or posterior endoscopic cervical discectomy technique. However, there is still a lack of high-quality randomized trials or meta-analyses. How about the recently developed endoscopic techniques for lumbar stenosis such as endoscopic laminotomy or bi-portal endoscopic surgery? Unfortunately, most studies demonstrating the effect of these techniques are still case series or technical notes. There is still a lack of high-quality comparative cohort studies on these new techniques. Although the current level of evidence of endoscopic spine decompression surgery is relatively low aside from transforaminal PELD, considering the current increasing interest in endoscopic spine surgery, high-quality clinical studies will be published in the near future.
Future perspectives
The development of the endoscopic spine surgery technique can be achieved through three aspects. First, advancement in the endoscopes, especially angled or steerable optics, will allow the practitioner to visualize all corners of the surgical field. Second, steerable instruments in burrs, punches, and forceps will make the decompression faster and safer for a wide range of surgical field. Finally, development of novel approaches will enable the treatment of new spinal levels such as high cervical or craniocervical junctions, thoracic and caudal levels, as well as lumbar and cervical levels.
The evolution of minimally invasive or endoscopic surgery is the main issue of current spine surgeons. The patients’ need for early recovery and return to a healthy lifestyle while maintaining a high quality of life will make the endoscopic surgery the mainstream of spine medicine. Furthermore, the increasing number of elderly patients will increase the demand for percutaneous endoscopic procedures with a minimal incision under local anesthesia. Regarding the current technological status, most lumbar herniated disc diseases and a considerable portion of spinal stenosis cases can be treated with endoscopic techniques. In the future, the definitive indications for endoscopic surgery will broaden rapidly. Eventually, most degenerative spine diseases will be treated with endoscopic surgery under local anesthesia in the near future.
Conclusions
Endoscopic spine surgery is an emerging technique in the field of MISS, providing benefits of minimal tissue trauma, low complication rates, and short recovery times. There are a variety of procedures for this technique that vary according to the endoscopes used, approaches taken, and spinal levels. Among them, the transforaminal PELD technique has been proven through randomized trials and meta-analyses as an excellent alternative surgical option. Regarding other endoscopic techniques, however, the current level of evidence is limited. Further technical development and high-quality studies are required to confirm the clinical relevance and effectiveness of endoscopic decompression technique for spinal disorders.
Acknowledgments
The author would like to thank Jae Min Son, and Ho Kim for their support and assistance with this review.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices 2012;9:361-6. [Crossref] [PubMed]
- Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices 2014;11:605-16. [Crossref] [PubMed]
- Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res 1986.37-43. [PubMed]
- Hijikata S. Percutaneous nucleotomy. A new concept technique and 12 years' experience. Clin Orthop 1989.9-23. [Crossref] [PubMed]
- Birkenmaier C, Komp M, Leu HF, et al. The current state of endoscopic disc surgery: review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician 2013;16:335-44. [PubMed]
- Ahn Y, Youn MS, Heo DH. Endoscopic transforaminal lumbar interbody fusion: a comprehensive review. Expert Rev Med Devices 2019;16:373-80. [Crossref] [PubMed]
- Kambin P, O'Brien E, Zhou L, et al. Arthroscopic microdiscectomy and selective fragmentectomy. Clin Orthop Relat Res 1998.150-67. [PubMed]
- Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine (Phila Pa 1976) 2002;27:722-31. [Crossref] [PubMed]
- Muramatsu K, Hachiya Y, Morita C. Postoperative magnetic resonance imaging of lumbar disc herniation: comparison of microendoscopic discectomy and Love's method. Spine (Phila Pa 1976) 2001;26:1599-605. [Crossref] [PubMed]
- Schick U, Döhnert J, Richter A, et al. Microendoscopic lumbar discectomy versus open surgery: an intraoperative EMG study. Eur Spine J 2002;11:20-6. [Crossref] [PubMed]
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002;51:S129-36. [Crossref] [PubMed]
- Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery 2002;51:S146-54. [Crossref] [PubMed]
- Hwa Eum J, Hwa Heo D, Son SK, et al. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine 2016;24:602-7. [Crossref] [PubMed]
- Choi CM, Chung JT, Lee SJ, et al. How I do it? Biportal endoscopic spinal surgery (BESS) for treatment of lumbar spinal stenosis. Acta Neurochir (Wien) 2016;158:459-63. [Crossref] [PubMed]
- Choi DJ, Jung JT, Lee SJ, et al. Biportal Endoscopic Spinal Surgery for Recurrent Lumbar Disc Herniations. Clin Orthop Surg 2016;8:325-9. [Crossref] [PubMed]
- Eun SS, Eum JH, Lee SH, et al. Biportal Endoscopic Lumbar Decompression for Lumbar Disk Herniation and Spinal Canal Stenosis: A Technical Note. J Neurol Surg A Cent Eur Neurosurg 2017;78:390-6. [Crossref] [PubMed]
- Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg 1993;78:216-25. [Crossref] [PubMed]
- Hermantin FU, Peters T, Quartararo L, et al. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 1999;81:958-65. [Crossref] [PubMed]
- Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976) 2006;31:E890-7. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931-9. [Crossref] [PubMed]
- Nellensteijn J, Ostelo R, Bartels R, et al. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J 2010;19:181-204. [Crossref] [PubMed]
- Cong L, Zhu Y, Tu G. A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur Spine J 2016;25:134-43. [Crossref] [PubMed]
- Li XC, Zhong CF, Deng GB, et al. Full-Endoscopic Procedures Versus Traditional Discectomy Surgery for Discectomy: A Systematic Review and Meta-analysis of Current Global Clinical Trials. Pain Physician 2016;19:103-18. [PubMed]
- Ruan W, Feng F, Liu Z, et al. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: A meta-analysis. Int J Surg 2016;31:86-92. [Crossref] [PubMed]
- Ahn Y, Lee SH, Park WM, et al. Posterolateral percutaneous endoscopic lumbar foraminotomy for L5-S1 foraminal or lateral exit zone stenosis. Technical note. J Neurosurg 2003;99:320-3. [PubMed]
- Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol 2005;17:641-61. [Crossref] [PubMed]
- Ahn Y, Oh HK, Kim H, et al. Percutaneous endoscopic lumbar foraminotomy: an advanced surgical technique and clinical outcomes. Neurosurgery 2014;75:124-33. [Crossref] [PubMed]
- Choi G, Lee SH, Raiturker PP, et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery 2006;58:ONS59-68. [PubMed]
- Ruetten S, Komp M, Godolias G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: prospective 2-year results of 331 patients. Minim Invasive Neurosurg 2006;49:80-7. [Crossref] [PubMed]
- Ahn Y. Percutaneous endoscopic cervical discectomy using working channel endoscopes. Expert Rev Med Devices 2016;13:601-10. [Crossref] [PubMed]
- Chiu JC, Clifford TJ, Greenspan M, et al. Percutaneous microdecompressive endoscopic cervical discectomy with laser thermodiskoplasty. Mt Sinai J Med 2000;67:278-82. [PubMed]
- Ahn Y, Lee SH, Chung SE, et al. Percutaneous endoscopic cervical discectomy for discogenic cervical headache due to soft disc herniation. Neuroradiology 2005;47:924-30. [Crossref] [PubMed]
- Ahn Y, Lee SH, Shin SW. Percutaneous endoscopic cervical discectomy: clinical outcome and radiographic changes. Photomed Laser Surg 2005;23:362-8. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop 2009;33:1677-82. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:940-8. [Crossref] [PubMed]
- Zdeblick TA, Zou D, Warden KE, et al. Cervical stability after foraminotomy. A biomechanical in vitro analysis. J Bone Joint Surg Am 1992;74:22-7. [Crossref] [PubMed]
- Grieve JP, Kitchen ND, Moore AJ, et al. Results of posterior cervical foraminotomy for treatment of cervical spondylitic radiculopathy. Br J Neurosurg 2000;14:40-3. [Crossref] [PubMed]
- Kim HS, Ju CI, Kim SW, et al. Huge Psoas Muscle Hematoma due to Lumbar Segmental Vessel Injury Following Percutaneous Endoscopic Lumbar Discectomy. J Korean Neurosurg Soc 2009;45:192-5. [Crossref] [PubMed]
- Ahn Y, Kim JU, Lee BH, et al. Postoperative retroperitoneal hematoma following transforaminal percutaneous endoscopic lumbar discectomy. J Neurosurg Spine 2009;10:595-602. [Crossref] [PubMed]
- Ahn Y, Lee HY, Lee SH, et al. Dural tears in percutaneous endoscopic lumbar discectomy. Eur Spine J 2011;20:58-64. [Crossref] [PubMed]
- Cho JY, Lee SH, Lee HY. Prevention of development of postoperative dysesthesia in transforaminal percutaneous endoscopic lumbar discectomy for intracanalicular lumbar disc herniation: floating retraction technique. Minim Invasive Neurosurg 2011;54:214-8. [Crossref] [PubMed]
- Ahn Y, Kim CH, Lee JH, et al. Radiation exposure to the surgeon during percutaneous endoscopic lumbar discectomy: a prospective study. Spine (Phila Pa 1976) 2013;38:617-25. [Crossref] [PubMed]
- Choi I, Ahn JO, So WS, et al. Exiting root injury in transforaminal endoscopic discectomy: preoperative image considerations for safety. Eur Spine J 2013;22:2481-7. [Crossref] [PubMed]
- Morgenstern R, Morgenstern C, Yeung AT. The learning curve in foraminal endoscopic discectomy: experience needed to achieve a 90% success rate. SAS J 2007;1:100-7. [Crossref] [PubMed]
- Lee DY, Lee SH. Learning curve for percutaneous endoscopic lumbar discectomy. Neurol Med Chir (Tokyo) 2008;48:383-8. [Crossref] [PubMed]
- Hsu HT, Chang SJ, Yang SS, et al. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 2013;22:727-33. [Crossref] [PubMed]
- Fan G, Gu X, Liu Y, et al. Lower Learning Difficulty and Fluoroscopy Reduction of Transforaminal Percutaneous Endoscopic Lumbar Discectomy with an Accurate Preoperative Location Method. Pain Physician 2016;19:E1123-34. [PubMed]
- Cheng J, Wang H, Zheng W, et al. Reoperation after lumbar disc surgery in two hundred and seven patients. Int Orthop 2013;37:1511-7. [Crossref] [PubMed]
- Gillard DM, Corenman DS, Dornan GJ. Failed less invasive lumbar spine surgery as a predictor of subsequent fusion outcomes. Int Orthop 2014;38:811-5. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine 2009;10:476-85. [Crossref] [PubMed]
- Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician 2015;18:61-70. [PubMed]
- Chen J, Jing X, Li C, et al. Percutaneous Endoscopic Lumbar Discectomy for L5S1 Lumbar Disc Herniation Using a Transforaminal Approach Versus an Interlaminar Approach: A Systematic Review and Meta-Analysis. World Neurosurg 2018;116:412-20.e2. [Crossref] [PubMed]
- Lee CH, Choi M, Ryu DS, et al. Efficacy and Safety of Full-endoscopic Decompression via Interlaminar Approach for Central or Lateral Recess Spinal Stenosis of the Lumbar Spine: A Meta-analysis. Spine (Phila Pa 1976) 2018;43:1756-64. [Crossref] [PubMed]


