A retrospective comparison of radiographic and clinical outcomes in single-level degenerative lumbar disease undergoing anterior versus transforaminal lumbar interbody fusion
Introduction
Lumbar spine fusion remains a treatment option for debilitating low back pain that fails to respond to conservative measures. A prior prospective randomized trial revealed that in selected patients, lumbar fusion outperforms nonsurgical treatments for chronic low back pain (1). This prior study included patients undergoing traditional posterolateral fusion (PLF), posterior lumbar interbody fusion (PLIF), and anterior lumbar interbody fusion (ALIF). However, no follow-up analysis was performed to distinguish outcomes among the different fusion techniques. To date, there remains a lack of randomized, prospective data comparing fusion techniques for lumbar degenerative disease. However, there is strong evidence to suggest that interbody fusion enhances fusion rates compared with posterolateral fusion alone (2).
Two of the most commonly performed lumbar interbody techniques are anterior lumbar interbody fusion (ALIF) and transforaminal lumbar interbody fusion (TLIF). The advantages of TLIF are the approach is well known to spine surgeons, it allows for direct decompression of the neural elements, and it is rarely associated with any major visceral complications. Several studies have shown that it has a better safety profile and improved outcomes compared with the more traditional posterior lumbar interbody fusion (PLIF) given less nerve root retraction, an ability to place the interbody graft more anteriorly, and opportunity for preservation of some posterior elements (3,4). ALIF has the advantage of avoiding the neural elements, complete removal of the disc, larger interbody graft surface area and a greater capacity for lumbar lordosis restoration. However, the need for an access surgeon, risk of major vascular complications, and retrograde ejaculation are all present with this approach (5).
Several retrospective series have sought to compare radiographic and clinical outcomes following ALIF and TLIF for degenerative lumbar disc disease or as long-segment support for deformity constructs (6-10). A recent meta-analysis comparing all lumbar interbody fusion techniques found similar fusion rates among ALIF and TLIF, with improvements in ODI scores for TLIF, and improved sagittal lordosis with ALIF (11). We sought to conduct a retrospective institutional study comparing radiographic and clinical outcomes in the intermediate term in a larger patient cohort with improved gradation in HRQOL scores than has previously been conducted. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-673).
Methods
Demographic and clinical variables
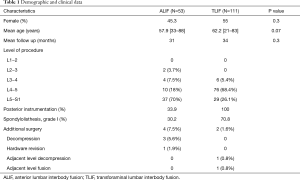
This is a retrospective review of 164 consecutive patients who underwent single-level ALIF or TLIF for lumbar degenerative disc disease at Rush University Medical Center (RUMC) over a 60-month period. All lumbar spine surgeries were queried between the years of 2013 and 2018. Patients were subsequently filtered by types of surgery where all non-ALIF or TLIF procedures were eliminated. Then, patients who had undergone ALIF or TLIF for more than one level were excluded from the study population. From this pool, diagnoses other than DDD were removed including spinal deformities, trauma, oncologic, grade II or higher spondylolisthesis, and revision cases. All remaining patients had symptomatic lumbar degenerative disc disease with none or grade I spondylolisthesis that had failed to respond to conservative measures. In addition, all patients consisted of pre-operative and post-operative imaging available for review. Next, the HRQOL survey status was assessed and the patients included had a minimum of one-year follow-up including complete HRQOL scores. Due to the small number of open TLIF patients, only the MIS-TLIF patients were selected in the final study population. The majority of patients with lumbar disc degeneration with disc herniation leading to foraminal stenosis ± radiculopathy were offered ALIF surgery. If rotational instability and/or a pars defect was suspected pre-operatively on flexion and extension upright x-ray films, posterior instrumentation was implemented for ALIF. Patients with degenerative disc disease with lower extremity radiculopathy leading to intractable leg pain and neurogenic claudication were offered the TLIF procedure. Members of our team reviewed each individual patient chart written by multiple physicians in order to ascertain the most accurate diagnosis of single-level DDD. In addition, complete operative notes were reviewed carefully to determine whether the operating neurosurgeon observed any additional conditions not apparent on imaging to reduce variables contributing to patient outcomes. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted following Rush University Medical Center Institutional Review Board approval (ORA# 19010702) and due to the nature of this retrospective study, individual consent for this analysis was waived.
Radiographic and HRQOL variables
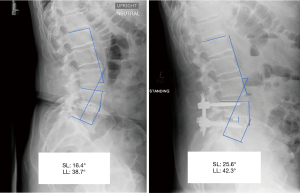
Pre-operative and post-operative upright films were used to assess segmental lordosis of the operated level and whole lumbar lordosis (Figure 1). Pre-operative upright films nearest to the surgery date were utilized as the baseline image for each patient. The inpatient post-operative upright radiographs immediately following surgery and at one-year follow-up radiographs were used for assessment. The lumbar lordosis was measured by using the method of Cobb to obtain the angle between the superior endplate of L1 and the superior endplate of S1. Segmental lordosis was similarly calculated between the superior endplate of the superior vertebral level and the inferior endplate of the inferior vertebral level involved in the interbody fusion. As for the L5-S1 segmental lordosis, the Cobb angle was measured between the superior endplate of the L5 vertebral level and the superior endplate of the S1 vertebral level as established in the literature (12). HRQOL scores were obtained using proprietary software (OBERD, Columbia, Missouri, USA) during office visits or remotely preoperatively and at regularly timed postoperative periods of 6 weeks, 3 months, 6 months, and 12 months then annually up to 5 years. The surveys included VAS-back, VAS-leg, Oswestry Disability Index (ODI), SF-12 Physical Health, SF-12 Mental Health, and SRS-30 Function/Activity. These surveys were administered by the neurosurgery staff clinic using the same description and explanation for each patient with no incentive to complete the questionnaires. We used previously established values for minimally clinically important difference (MCID) in lumbar spine surgery to assess the percentage of patients in each cohort meeting these parameters (13,14).
Surgical technique
All ALIFs are performed in conjunction with the general surgery service for access. The anterior lumbar space is approached via a left-sided paramedian retroperitoneal approach. After access is obtained and confirmation of the target level is made with fluoroscopy, the anterior longitudinal ligament is incised, and a discectomy is performed. An interbody device is placed after trialing and the incision is closed. Depending on surgeon’s preference, ALIF was either performed alone or with posterior instrumentation. In the ALIF posterior fixation cohort, no compression of construct was performed to further the lordosis of patients. In addition, an expandable cage was inserted for those patients who presented with severe loss of disc height at the level of operation. TLIFs were performed in a minimally-invasive fashion as is well-described in the literature (15). The specific approach was surgeon-dependent. Percutaneous screws with fixed diameter access port was utilized and bilateral pedicle fixation was performed as is usual with this approach. Expandable cage was implemented for those patients with severe disc collapse and limited by anatomical access to intervertebral disk space.
Statistical analysis
Independent groups t-tests were performed to compare means of radiographic and HRQOL outcomes. A Fisher’s exact test was used to compare gender frequencies in both cohorts. Inter-observer reliability was assessed using intraclass correlation coefficients between two observers, a neurosurgery spine fellow and a senior neurosurgery resident. Each of these members of the team were blinded to patient information and the type of procedure being performed while conducting this task. We interpreted coefficients between 0.61−0.80 as substantial agreement and above 0.81 as almost perfect agreement (16).
Results
Demographics
A total of 164 patients were included in the study. There were 53 patients who underwent ALIF and 111 patients who underwent TLIF. The mean age of patients in the ALIF cohort was 57.9 years and 62.2 years for the TLIF cohort. Female patients comprised 45.3% of ALIF patients and 55% of TLIF patients. There was no significant difference in age, gender, or follow-up duration between the two cohorts. The mean follow-up for the ALIF group was 31 months and 34 months for the TLIF group. All patients underwent a single-level ALIF or TLIF surgery with the majority of procedures occurring at the level of L4−5 and L5−S1 in both groups (Table 1).
Full table
Peri-operative and radiographic outcomes
To determine the peri-operative outcomes, the study focused on the estimated blood loss, hospital length of stay post-surgery, and the complications associated with each surgical intervention. The mean estimated blood loss (EBL) was 70.5 (95% CI: 56.4, 84.8) mL for the ALIF group and 147.7 (95% CI: 120.9, 174.7) mL for the TLIF group (P<0.05). The mean length of stay (LOS) was 2.9 (95% CI: 2.3, 3.5) days for the ALIF group and 3.5 (95% CI: 3.1, 3.9) days for the TLIF group (P=0.12). In terms of complications, there were no major complications noted in both groups while 3 minor complications were shown in the ALIF group and 13 minor complications for the TLIF group (Table 2). Upon review, the minor complications were found to be post-operative fever, ileus, urinary retention, and deep venous thrombosis. These complications were classified as previously described in the literature (17). There was a total of 8 unintended durotomies (7.2%), all of which occurred in the TLIF cohort that were repaired intraoperatively without subsequent complications.

Full table
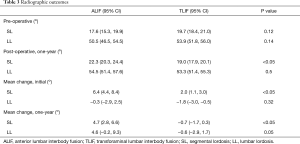
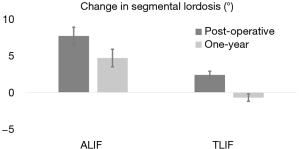
Segmental and lumbar lordosis of patients undergoing ALIF and TLIF surgeries were analysed radiographically. Pre-operative segmental and lumbar lordosis was found to be similar between groups [ALIF 17.6° (95% CI: 15.3, 19.9) vs. TLIF 19.7° (95% CI: 18.4, 21.0), P=0.12; ALIF 50.5° (95% CI: 46.5, 54.5) vs. TLIF 53.9° (95% CI: 51.8, 56.0), P=0.14]. In addition, post-operative lumbar lordosis was not significantly different between the ALIF and TLIF patients (P=0.5). However, the post-operative segmental lordosis was significantly different at follow-up [ALIF 22.3° (95% CI: 20.3, 24.4) vs. TLIF 19.0° (95% CI: 17.9, 20.1), P<0.05]. The mean segmental and lumbar lordosis was also analysed to determine which surgical intervention results in a greater degree of change. While the change in segmental lordosis was significantly greater in ALIF patients immediately following surgery [ALIF 6.4° (95% CI: 4.4, 8.4) vs. TLIF 2.0° (95% CI: 1.1, 3.0), P<0.05], the change in lumbar lordosis was not found to be significantly different [ALIF −0.3° (95% CI: −2.9, 2.5) vs. TLIF −1.8° (95% CI: −3.0, −0.5), P=0.32] (Table 3). The radiographic result for the change in segmental lordosis remained significantly different between both groups at one year of follow-up (Figure 2). Given that the majority of surgeries occurred at L4−5 and L5−S1, we examined the magnitude of the change in segmental lordosis on a level-by-level analysis. We found a significantly greater change at L5−S1 in the ALIF cohort (ALIF 9.6° vs. TLIF 3.4°, P<0.005). At L4−5, while the ALIF cohort had a greater mean change, it did not reach statistical significance (ALIF 4.3° vs. TLIF 1.4°, P=0.34). The majority of ALIF and TLIF patients were instrumented with static spacers and the ALIF interbody spacers had a higher lordotic angle profile but a similar height profile with TLIF interbody spacers (Table 1). The lordotic angles of the ALIF interbody spacers ranged from 6−15° while the TLIF interbody spacers ranged from 4−8°. We focused on the effects of static and expandable interbody devices on TLIF surgery and how it influences segmental lordosis. The use of an expandable cage did not significantly alter the segmental lordosis in comparison to use of static cages at one year (Expandable 17.2° vs. Static 19.7°, P=0.09) (Figure 2).
Full table
Intraclass correlation coefficients were obtained to assess inter-observer reliability between two observers. All the radiographic assessments resulted in an ICC coefficient above 0.80 indicating excellent reliability (Table 4).
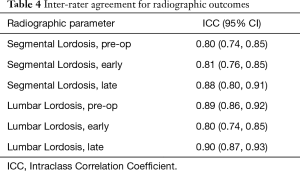
Full table
Clinical outcomes
A battery of pre-operative and post-operative surveys were used to assess VAS-back, VAS-leg, ODI, SF-12 Physical Health, SF-12 Mental Health, and SRS-30 Function/Activity scores. First, we evaluated pre-operative baseline patient-reported outcomes and found VAS-leg to be significantly higher in the TLIF cohort [ALIF 3.9 (95% CI: 2.6, 5.2) vs. TLIF 6.1 (95% CI: 5.4, 6.7), P<0.05] while SF-12 Mental Health was found to be significantly lower in the TLIF cohort [ALIF 53.3 (95% CI: 49.7, 56.8) vs. TLIF 47.8 (95% CI: 44.8, 50.9), P<0.05]. Other patient-reported outcomes were statistically similar between both groups. Next, we sought to determine the difference in magnitude of change resulting from ALIF or TLIF in these cohorts by subtracting their pre-operative baseline assessment from their one-year post-operative assessment. Then, we compared whether the magnitudes of change were statistically different between the two cohorts. At one-year post-surgical assessment, the change in patient-reported outcomes was significantly greater for VAS-leg and ODI in the TLIF cohort compared to the ALIF cohort (P<0.05). In terms of back pain, the VAS-back survey did not reveal any significant difference between the two surgical interventions (P=0.06). On further analysis, SF-12 Physical Health and SRS-30 Function/Activity scores did not demonstrate any statistical difference in the magnitude of the changes between the two groups. The only significant differences that favored the ALIF cohort was their significantly greater improvement in SF-12 Mental Health scores (P<0.05) (Table 5). We found MCID was achieved in a majority of patients in both cohorts across all HRQOL outcomes (Table 6).
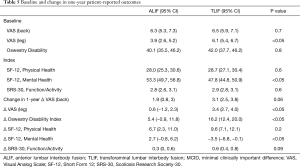
Full table
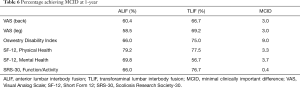
Full table
In the ALIF cohort, three patients required additional surgery to decompress the index level of surgery and one patient required a hardware revision. The hardware revision was performed due to evidence of subsidence of the spacer after the index surgery. Two patients developed adjacent segment disease in the TLIF cohort, one of which underwent adjacent level fusion and the other patient underwent an adjacent level decompression (Table 1). Both patients who developed adjacent segment disease had intact adjacent segments prior to index surgery. While this study was not designed to assess fusion rates due to the absence of consistent CT imaging at 1-year follow-up, no patient in either group required reoperation specifically for symptomatic pseudarthrosis.
Discussion
ALIF and TLIF are known to be effective treatments for degenerative lumbar disc disease (DDD) (18). With the benefit of accessing the intervertebral space directly from the midline, ALIF has been recognized to provide an advantage over TLIF in gaining lumbar or segmental lordosis (6,7,9). This surgical intervention allows for a larger cage insertion permitting a greater correction of lordosis. The TLIF technique allows for unilateral access to the intervertebral foraminal space reducing the potential damage to important neural structures such as the dura and nerve roots (15). More recently, the minimally-invasive surgical approach of TLIF has propelled the prevalence of this surgery in treating DDD by minimizing paraspinal muscle injury, bleeding, and post-operative recovery time (19,20).
This manuscript addresses both radiological and functional outcomes in patients after ALIF or TLIF surgery with minimum of one-year follow-up. In the current literature, there are only a few studies presenting both radiological and functional outcomes of patients undergoing ALIF versus TLIF surgery (6-9,21). These studies have indicated a superiority either in lumbar lordosis or segmental lordosis in patients who underwent ALIF compared to TLIF (6,7,9,21). Crandall and Revella et al. failed to demonstrate a significant difference in lumbar lordosis between patients of ALIF and TLIF for degenerative lumbar scoliosis (8). Although Watkins et al. did not include clinical outcomes in their study, the authors established a significant difference between the ALIF and TLIF cohorts in restoring lumbar lordosis (10). Our results indicate a significantly greater change in lumbar lordosis and segmental lordosis after ALIF surgery which agrees with previous studies (6,7). Interestingly, we found that while there remained a significant difference in changes in segmental lordosis between ALIF and TLIF cohorts at one year, both groups had a reduction in their correction over time when comparing their immediate post-operative standing x-rays with those obtained at one year. For the TLIF cohort, this change was reflected in a slight segmental lordosis turning into a slight kyphosis at one year. This aligns with the understanding that the magnitude of segmental change only decreases with time after surgery. The effect of static and expandable interbody devices on TLIF was also examined. When comparing the application of the two cage types, our study did not demonstrate any significant difference in segmental lordosis. There are only two primary studies in the literature that directly compare static with expandable interbody devices (22,23). Yee et al. analyzed the segmental and lumbar lordosis in single-level TLIF patients and concluded that there is no apparent statistical significance at one month or one year post-operatively (22). Hawasli et al. focused on MIS-TLIF surgeries and discovered that expandable cages yielded a greater increase in segmental lordosis compared to static cages. In terms of lumbar lordosis, Hawasli et al. did not indicate any significant difference between the two types of interbody devices (23). In 2019, Alvi et al. conducted a meta-analysis comparing static and expandable interbody devices in MIS-TLIF patients in which they did not observe any significant difference in clinical and radiographic assessments (24). Further studies are warranted to determine the efficacy of the expandable interbody devices, nevertheless, our study agrees with the previous literature which suggests that expandable cages do not restore segmental lordosis to a greater degree than static cages.
While most previous studies allude to the benefits of ALIF radiologically, the functional outcomes have resulted in no statistical significance. Improvements were shown in ALIF and TLIF cohorts individually; however, there was no significant difference when directly comparing the two surgical interventions (6-9,21). Hsieh et al. noted at the end of two years they found no clinically significant VAS score difference between the two corrective surgeries (6). In addition, Kim et al. revealed that there were no statistically significant improvements in either VAS or ODI when comparing ALIF with TLIF (7). Other studies have demonstrated the same (8,9). In 2010, the same authors, Kim et al., concluded that the ODI scores improved for the TLIF cohort when focusing solely on surgeries at the level of L4-5 while both VAS and ODI scores at the level of L5-S1 did not show any improvements (21). Our retrospective study is unique in that a significant difference was observed in the functional outcome of patients when comparing ALIF with TLIF. To the authors’ knowledge, there are no major studies that have resulted in similar clinical outcomes except for one ODI outcome measure at the level of L4-5 in the study noted above (21). Our study strongly suggests that patients who have undergone TLIF surgery have larger decreases in ODI scores at one year or greater, suggesting a better functional outcome compared to ALIF patients. Although the change in VAS-leg score was significant at one year, this decrease in VAS-leg score can be attributed to the statistically different baseline VAS-leg scores. In fact, when calculating the final VAS-leg score at one year, the scores do not reach clinical significance. The ODI, however, may be a better representation of the superiority of TLIF in functional outcome as the baseline ODI was similar between the two cohorts. In addition, the change in ODI and thus the final ODI reached a clinically significant difference. It is important to remember that changes in HRQOL scores do not necessarily correlate to MCID. A recent study comparing surgical effectiveness of ALIF, TLIF, and LLIF at one year found that VAS-back, VAS-leg, and ODI scores that reached MCID in the first year were more likely to achieve MCID threshold at two years. More specifically, across patient cohorts, the MCID threshold at two years was 12- to 14-fold more likely to be reached for ODI which was greater than VAS-back and leg scores (13). Not only does this demonstrate that ODI changes translate to a more meaningful long-term improvement and recovery, but in terms of our study, it suggests that TLIF remains a better procedure for functional outcome in patients where an ALIF or TLIF may be considered. In terms of other HRQoL outcomes, there were no significant differences found between cohorts in SF-12 Physical Health and SRS-30 Function/Activity scores. Of note, SF-12 Mental Health score was the only outcome that had a greater improvement in the ALIF cohort. When the pre-operative SF-12 Mental Health scores were reviewed, it was found that the ALIF cohort started with a significantly higher mental health score. At least one prior study examining the effect of mental health on patient-reported outcomes in cervical spine surgery revealed that patients with higher pre-operative mental health scores improved more so than those with lower scores (25). This may partly explain why the ALIF cohort had greater improvements in mental health but would not explain the remainder of the outcome findings. Ultimately, the significance of this finding is unclear and will need further study.
Every surgical intervention consists of peri-operative and post-operative complications and TLIF and ALIF were no exceptions. Due to its angle of approach, ALIF is associated with vascular injury, visceral organ damage, and retrograde ejaculation. TLIF is known to produce significant paraspinal muscle injury due to its mandated retraction during surgery and dorsal root ganglia injury (18). It is difficult to assess the various types of complications and its severity as there are limited studies available in the literature. Nonetheless, one recent meta-analysis did note a greater rate of dural injury in the TLIF population while the ALIF cohort endured more vascular injury (26). In this current study, complication rates were shown to be similar. No major complications were evident in either surgery, but a few minor complications were noted in each cohort. Additionally, when comparing EBL and LOS between the two types of surgeries, MIS TLIF was observed to have significantly greater blood loss; however, while length of stay on average was longer for MIS TLIF, it was not statistically significant. Prior studies have shown varying effects of these two values with the general consensus indicating a shorter hospital stay for TLIF patients (26).
There are several limitations to this study. During the retrospective analysis of patient charts, more TLIF patients were found to fit the inclusion criteria compared to ALIF. In addition, the TLIF surgery was recommended more by certain surgeons to correct DDD as these surgeons are known to prefer a certain intervention. It is also important to note that the majority of ALIF surgeries were performed at L5−S1 while most TLIF surgeries were operated on at L4−5. The variability of the surgeon’s technique and experience, the types of implant utilized, and other co-morbidities of patients may be other sources of bias and confounding. The implants used were variable ranging from non-expandable to expandable and limited by the anatomy of each patient resulting in a highly unpredictable outcome in lordosis.
With a carefully selected study population and minimization of potential bias despite being a retrospective study, the generalizability of the study is robust. The study population comprised of patients diagnosed with a single-level degenerative disc disease (DDD) with or without grade I spondylolisthesis. The patients’ ages widely ranged from 21 to 88 with mean age, gender, and follow-up duration which did not show any significant differences between the two cohorts. In addition, patients from multiple neurosurgeons participated in this study rather than a single pool of patients from a single neurosurgeon allowing better generalizability for interpretation. Furthermore, DDD is known to account for 266 million patients or 3.63% worldwide. More specifically, 39 million or 0.53% of the population worldwide are found to have spondylolisthesis (27). As the population ages, it is difficult to deny the increasing demand for therapeutic measures for these patients. Although this study was conducted at a single institution, we provide profound impact on spine surgeons who may need assistance in choosing the proper surgical technique for treatment of DDD. The two procedures presented in this study are one of the most common surgical techniques currently used to treat DDD. Not only are these surgeries highly prevalent in other institutions, but especially now that MIS is becoming a household technique among spine surgeons, our MIS TLIF cohort can offer further insight into a neurosurgeon’s decision when treating DDD.
Conclusions
ALIF and TLIF are commonly recommended to correct degenerative lumbar disc disease. This current study addresses both the radiological and functional outcomes of patients who either underwent single-level ALIF or TLIF surgery. The radiological assessment of patients aligned with previous studies in showing ALIF as a superior method of increasing lumbar and segmental lordosis. In contrast, TLIF was utilized more commonly in patients with higher pre-operative VAS-leg pain scores and thus, showed a greater magnitude of VAS-leg pain improvement. However, TLIF also demonstrated a greater improvement in ODI scores despite similar baseline scores between the groups, suggesting a possible enhanced functional outcome.
Acknowledgments
A modified portion of the manuscript has been presented previously at the AANS/CNS Annual Spine Summit 2020 as an oral presentation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-673
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-673
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-673). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted following Rush University Medical Center Institutional Review Board approval (ORA# 19010702) and due to the nature of this retrospective study, individual consent for this analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976) 2001;26:2521-32; discussion 2532-4. [Crossref] [PubMed]
- Mummaneni PV, Dhall SS, Eck JC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine 2014;21:67-74. [Crossref] [PubMed]
- Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976) 2001;26:567-71. [Crossref] [PubMed]
- Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009;2:118-26. [Crossref] [PubMed]
- Sasso RC, Best NM, Mummaneni PV, et al. Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine (Phila Pa 1976) 2005;30:670-4. [Crossref] [PubMed]
- Hsieh PC, Koski TR, O'Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 2007;7:379-86. [Crossref] [PubMed]
- Kim JS, Kang BU, Lee SH, et al. Mini-transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation: a comparison of surgical outcomes in adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech 2009;22:114-21. [Crossref] [PubMed]
- Crandall DG, Revella J. Transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion as an adjunct to posterior instrumented correction of degenerative lumbar scoliosis: three year clinical and radiographic outcomes. Spine (Phila Pa 1976) 2009;34:2126-33. [Crossref] [PubMed]
- Dorward IG, Lenke LG, Bridwell KH, et al. Transforaminal versus anterior lumbar interbody fusion in long deformity constructs: a matched cohort analysis. Spine (Phila Pa 1976) 2013;38:E755-62. [Crossref] [PubMed]
- Watkins RGt. Sagittal alignment after lumbar interbody fusion: comparing anterior, lateral, and transforaminal approaches. J Spinal Disord Tech 2014;27:253-6. [Crossref] [PubMed]
- Teng I, Han J, Phan K, et al. A meta-analysis comparing ALIF, PLIF, TLIF and LLIF. J Clin Neurosci 2017;44:11-7. [Crossref] [PubMed]
- Kim SB, Jeon TS, Heo YM, et al. Radiographic results of single level transforaminal lumbar interbody fusion in degenerative lumbar spine disease: focusing on changes of segmental lordosis in fusion segment. Clin Orthop Surg 2009;1:207-13. [Crossref] [PubMed]
- Adogwa O, Elsamadicy AA, Han JL, et al. Do measures of surgical effectiveness at 1 year after lumbar spine surgery accurately predict 2-year outcomes? J Neurosurg Spine 2016;25:689-96. [Crossref] [PubMed]
- Diaz-Arribas MJ, Fernandez-Serrano M, Royuela A, et al. Minimal Clinically Important Difference in Quality of Life for Patients With Low Back Pain. Spine (Phila Pa 1976) 2017;42:1908-16. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360-3. [PubMed]
- Campbell PG, Yadla S, Malone J, et al. Complications related to instrumentation in spine surgery: a prospective analysis. Neurosurg Focus 2011;31:E10 [Crossref] [PubMed]
- Ajiboye RM, Alas H, Mosich GM, et al. Radiographic and Clinical Outcomes of Anterior and Transforaminal Lumbar Interbody Fusions: A Systematic Review and Meta-analysis of Comparative Studies. Clin Spine Surg 2018;31:E230-8. [Crossref] [PubMed]
- Hee HT, Castro FP Jr, Majd ME, et al. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord 2001;14:533-40. [Crossref] [PubMed]
- Scheer JK, Auffinger B, Wong RH, et al. Minimally Invasive Transforaminal Lumbar Interbody Fusion (TLIF) for Spondylolisthesis in 282 Patients: In Situ Arthrodesis versus Reduction. World Neurosurg 2015;84:108-13. [Crossref] [PubMed]
- Kim JS, Lee KY, Lee SH, et al. Which lumbar interbody fusion technique is better in terms of level for the treatment of unstable isthmic spondylolisthesis? J Neurosurg Spine 2010;12:171-7. [Crossref] [PubMed]
- Yee TJ, Joseph JR, Terman SW, et al. Expandable vs. Static Cages in Transforaminal Lumbar Interbody Fusion: Radiographic Comparison of Segmental and Lumbar Sagittal Angles. Neurosurgery 2017;81:69-74. [Crossref] [PubMed]
- Hawasli AH, Khalifeh JM, Chatrath A, et al. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg Focus 2017;43:E10 [Crossref] [PubMed]
- Alvi MA, Kurian SJ, Wahood W, et al. Assessing the Difference in Clinical and Radiologic Outcomes Between Expandable Cage and Nonexpandable Cage Among Patients Undergoing Minimally Invasive Transforaminal Interbody Fusion: A Systematic Review and Meta-Analysis. World Neurosurg 2019;127:596-606.e1. [Crossref] [PubMed]
- Diebo BG, Tishelman JC, Horn S, et al. The impact of mental health on patient-reported outcomes in cervical radiculopathy or myelopathy surgery. J Clin Neurosci 2018;54:102-8. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Ravindra VM, Senglaub SS, Rattani A, et al. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Global Spine J 2018;8:784-94. [Crossref] [PubMed]