Early clinical and radiological results of unilateral posterior pedicle instrumentation through a Wiltse approach with lateral lumbar interbody fusion
Introduction
Lateral lumbar interbody fusion (LLIF) is a relatively novel technique in spine surgery that accesses the anterior and middle vertebral columns for interbody fusion via a direct lateral transpsoas approach. LLIF offers several advantages over posterolateral fusion, including indirect foraminal decompression and correction of spinal alignment (1-5). Compared to traditional open interbody fusion techniques, such as anterior lumbar interbody fusion (ALIF) and posterior lumbar interbody fusion (PLIF), LLIF is thought to be associated with decreased blood loss, early patient mobilization, and decreased hospital stay (5).
The biomechanical characteristics of LLIF cages differ from those used in traditional open spine procedures. In fact, commercially available LLIF cages are designed to rest on vertebral apophyseal bone, which is stronger than central cancellous bone that ALIF and PLIF cages are designed to rest on, thereby improving graft-host interface (6,7). Since the LLIF cage spans the entire vertebra, there is better distribution of compressive loads as opposed to the smaller PLIF cages, which provides improved mechanical support to maintain interbody height and minimize subsidence (8). Additionally, there is preservation of the anterior and posterior longitudinal ligaments, which offers better postoperative stabilization (5,9). We hypothesized that reducing supplemental fixation to LLIF cages may significantly reduce operative time, blood loss, and surgical invasiveness with minimum tradeoff in patient outcome.
The purpose of this study was to assess the clinical and radiographic outcomes of 44 patients who underwent single-level LLIF augmented with unilateral pedicle screw instrumentation without the use of plating of the anterior column.
Methods
This study was approved by the Institutional Review Board. This was a retrospective review of prospectively collected data. Between September 2007 and July 2013, the senior surgeon performed a total of 181 cases using the XLIF cage (XLIF, NuVasive Inc., San Diego, CA, USA). There were 66 patients who underwent single level interbody fusion, of which 13 patients had unilateral pedicle screw fixation with anterior column plate, 46 patients had unilateral pedicle screw fixation without anterior column plate, and 7 patients had bilateral pedicle screw fixation. The senior surgeon determined that bilateral pedicle screws were appropriate in 3 patients with osteoporosis and vertebral body compression fractures, 2 patients with concurrent neoplastic processes, 1 patient with revision surgery, and 1 patient requiring extensive posterior decompression.
Of the 46 patients with unilateral pedicle screw fixation without anterior column plating, 2 patients were excluded due to incomplete data, leaving 44 total patients included in the final analysis. The most frequently addressed surgical level was the L4–5 motion segment (n=34, 77.3%), followed by L3–4 (n=8, 18.2%), L2–3 and L1–2 (n=1, 2.3%, each). Diagnoses indicating a fusion procedure were spondylolisthesis with central canal stenosis in 38 patients (86.4%), degenerative disc disease with foraminal stenosis in 4 patients (9.1%), and scoliosis with central canal stenosis in 2 patients (4.5%).
Data on demographics, comorbidities, clinical assessment, perioperative details, and complications were assessed. Oswestry disability index (ODI), short form-12 (SF-12), and visual analog scale (VAS) back scores were obtained before surgery and at regular follow-up visits using InQuiry 0.151s (Phoenix Medcom Inc., Cortlandt Manor, NY, USA) electronic medical record software and office charts.
Evaluation of fusion status, cage migration, and subsidence of the interbody cage into the superior and inferior endplates was performed using anteroposterior (AP) and lateral (Lat) plain radiographs by one musculoskeletal fellowship-trained attending radiologist. Fusion status criteria were defined as bridging trabeculae crossing the adjacent vertebral bodies either through or around the implants and an absence of radiolucent lines around more than 50% of either of the implant surfaces. Fusion criteria of less than 5° of angular motion and less than or equal to 3 mm of translation were evaluated on flexion/extension (flex/ex) views if available (10). Subsidence was classified as described by Marchi et al., with Grade 0 if there was 0–24% loss of post-operative disc height as an average of anterior and posterior disc heights, Grade 1: 25–49%, Grade 2: 50–74%, and Grade 3: 75–100% (10). Radiographs were obtained using Picture Archiving and Communication System (Version 16.1.22.1566, McKesson, San Francisco, California, USA).
Surgical technique
LLIF was performed using the technique performed similarly to the technique described by Ozgur et al., modified using a single-incision only as described in prior manuscripts (5,11). The LLIF was performed through a right-sided approach in 6 cases and through a left-sided approach in 38 cases (5). Cage dimensions were available for 41 patients (93%), with cages of 18 mm width used in 35 cases (85%) and 22 mm used in 6 cases (15%). Recombinant human bone morphogenic protein-2 (rhBMP-2) with Grafton demineralized bone matrix (DBM) putty (Osteotech Inc., Eatontown, NJ, USA) was used as the interbody graft in 32 patients, autologous iliac crest bone graft with Grafton DBM putty in 11 patients, and Grafton DBM putty alone in 1 patient.
Posterior surgery was performed afterwards using a standard paramedian Wiltse approach (12). Microsurgical hemilaminectomy decompression was performed in 24 patients. Patients who did not receive decompressions were either felt to not have significant central stenosis or foraminal stenosis that was adequately addressed by indirect decompression. Unilateral pedicle screw instrumentation was placed (24 on the right side, 20 on the left side) using direct visualization of bony landmarks and fluoroscopy (Figure 1). Supplemental unilateral concomitant posterolateral fusion was performed on all patients by using decortication of the transverse processes, augmented with autologous bone graft combined with Grafton DBM putty in 31 patients (70.5%) and autologous bone graft/ DBM putty/ rhBMP-2 combination in 13 patients (29.5%).

Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD). Paired two-tailed students t-tests were used to compare pre-operative and post-operative clinical outcomes. Fusion rates were compared for rhBMP-2 vs. non-rhBMP-2 constructs using Fisher’s exact test. Clinical outcomes were compared in patients with successful interbody fusion vs. patients with pseudarthrosis by Mann-Whitney U-test. Statistical analysis was performed using SPSS 20.0.0 (IBM, Armonk, New York, USA). Alpha values less than 0.05 were considered statistically significant.
Results
Patient demographics
The patient cohort included 15 men and 29 women, with an average age of 60.8±10.6 years (range, 32–79 years). Average body mass index (BMI) of patients was 29.7±6.4 kg/cm2 (range, 20.2–44.6 kg/cm2). Pre-operative narcotics were used by 16 patients, and previous lumbar surgery was performed in 11 patients.
Perioperative course
The mean operative time was 195.7±36.9 minutes (range, 111–295 minutes), with an average estimated blood loss of 159.3±90.8 cc (range, 50–500 cc). There were no cases of intra-operative complications, and no patients received a transfusion during the procedure or the postoperative hospital stay. Average length of hospital stay was 4.2±2.5 days (range, 2–13 days).
Clinical indices
Post-operative clinical outcome instruments were taken after an average of 11.7±11.5 months (range: 2–51 months) after the index surgery. There were 21 patients (48%) with greater than 6 months of follow-up. VAS scores improved from pre-operatively (7.8±1.7 out of 10) to final follow-up (3.3±3.6 out of 10), a statistically significant difference of 4.5 points (P<0.001). ODI scores also improved from pre-operatively (46.8±13.2 out of 100) to final follow-up (25.7±15.2 out of 100), a significant improvement of 21.1 (P<0.001). SF-12 Physical Health Composite Score (PCS) improved from pre-operatively (29.6±5.9) to final follow-up (35.3±9.5), a significant improvement of 5.7 points (P<0.001). SF-12 Mental Health Composite Score (MCS) improved from pre-op (46.5±10.7) to final follow-up (50.5±12.3), but this 4.0 point improvement was not statistically significant (P=0.053) (Table 1).
Full table
Radiographic evaluation
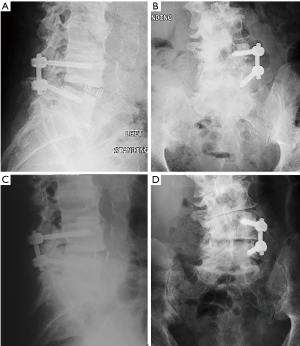
Average radiographic follow-up was 15.4±13.2 months (range: 1.3–66.3 months). There were 39 patients with final follow-up radiographs acquired after minimum half-year post-op. Out of these 39 patients, there were 35 cases of successful fusion (90%). There was no significant difference in rates of fusion between cages with rhBMP-2 (27/29, 93%) versus without rhBMP-2 (8/10, 80%) (P=0.267). There was not a significant difference in clinical scores between patients with successful fusion versus unfused at final radiographic follow-up (SF12 PCS P=0.641, SF12 MCS P=0.801, ODI P=0.860, VAS P=0.742). Subsidence of the adjacent cranial vertebra was scored a Grade 0 in 37 patients (95%) and as Grade 1 in 2 patients (5%). Subsidence of the adjacent caudal vertebra was scored as Grade 0 in 39 patients (100%). There was cage migration ≥3 mm in 6 cases (5 ventral and 1 dorsal) by measurement of cage marker position relative to adjacent vertebral bodies (Figure 2).
Complications
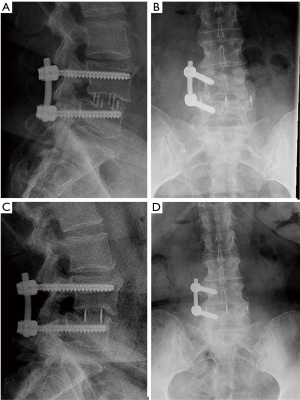
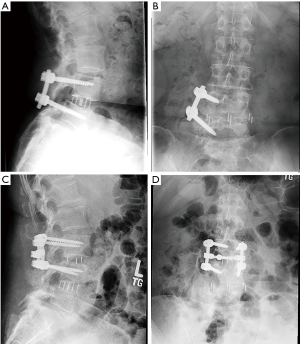
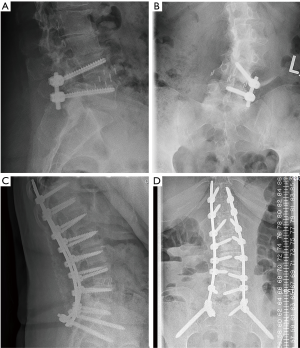
There were 7 (16%) cases of neurological deficits. One patient experienced quadriceps weakness, graded according to the medical research council (MRC) system, which improved from 1/5 MRC to 4/5 MRC after 1 year of physical therapy. A large anterior branch of this patient’s lumbar plexus was encountered during the surgical approach that needed to be mobilized for access to the disc space. During follow-up, the patient experienced quadriceps palsy, with loss of light touch sensation of the ipsilateral L3 dermatomal region. After 5 days, there was return of sensation, along with hyperesthesia and neuropathic pain. The patient’s iliopsoas and quadriceps motor power was 2/5 MRC, and 1/5 MRC respectively, which improved to 4+/5 MRC after 1 year of physical therapy. Six patients reported transient post-operative paresthesia that resolved over the course of 6 months. One patient experienced a L5 cage subsidence-related fracture that was treated non-operatively (Figure 3). Two (5%) patients required revision decompression for adjacent segment disease (ASD). One patient developed ASD 34 months after index surgery on the cranial adjacent level, which was revised with removal of prior pedicle screw instrumentation and insertion of an interbody fusion cage with bilateral pedicle screw fixation (Figure 4). Another patient developed ASD 35 months after index surgery on the cranial adjacent level, which was revised with a laminotomy. One patient (2%) developed progressive degenerative scoliosis that required revision with removal of prior instrumentation followed by a T11-pelvis bilateral pedicle screw fixation (Figure 5).
Discussion
The LLIF technique was introduced in 2001 by Ozguret al. as a modification of the retroperitoneal approach to the lumbar spine (5). The potential advantages of the LLIF technique include less invasive access, the preservation of stabilizing ligaments, restoration of intervertebral disc height with indirect decompression of the intervertebral foramina, and satisfactory fusion rates (5,9,13,14). Because of the additional stabilization afforded by the LLIF procedure, the senior surgeon of this present study was encouraged to explore the impact of less extensive posterior instrumentation with the idea that unilateral pedicle screw placement may result in less operative time, less blood loss, and lower costs without compromising clinical and radiographic outcomes. Our results demonstrate that good clinical and radiographic outcomes may be achieved with the LLIF approach supplemented with a unilateral pedicle screw construct inserted through a Wiltse approach.
To the authors’ knowledge, there are no published clinical studies examining unilateral pedicle screw fixation in the setting of LLIF without supplemental plate fixation of the anterior column. A cadaveric study by Cappuccino et al. demonstrated that interbody fusion supplemental with bilateral pedicle screw fixation provided the greatest reduction in range of motion (15). Compared to LLIF with unilateral pedicle screw fixation, LLIF with bilateral pedicle screw fixation was associated with a significant reduction in flexion-extension (13.0% versus 20.4%), lateral bending (14.4% versus 21.6%), and axial rotation (41.7% versus 51.3%) (15). However, several authors reported the achievement of high fusion rates using LLIF with less stable posterior constructs. Kepler et al. reported a 100% fusion rate after 1 year follow-up in patients who had undergone unilateral pedicle screw fixation following LLIF with supplemental anterior plate fixation, while Marchi et al. reported an overall 91% fusion rate in stand-alone LLIF (10,16). The fusion rate of this current study (90%) is comparable to the fusion rates reported by these two studies. A randomized control study may better clarify the impact of supplemental fixation following LLIF on fusion rates by removing possible study-center related bias.
In addition to supplemental fixation, cage shape and size may also important considerations for optimizing the contact area between implant and endplate to obtain solid fusion. Le Huec found that a stand-alone cylindrical interbody cage was not inherently stable, and that a lateral plate was required for stability (17). Cylindrical cages are thought to be inherently unstable because of their limited implant-endplate contact area. In contrast, large rectangular LLIF cages were shown to provide much greater implant-endplate contact area. In terms of cage size, a study by Marchi et al. on stand-alone LLIF cages reported an overall 91% fusion rate using cages with width of 18 and 22 mm (10). In this present study, the majority of cages were of 18 mm width (85%). Pimenta et al. have found that the larger, wider 26 mm cages are biomechanically more stable than the smaller 18 mm cages (8). A wider cage may achieve higher rates of fusion when the unilateral pedicle screw construct is being considered for use with LLIF. Further studies assessing the impact of LLIF cage sizes on fusion rates should be performed using CT imaging.
There is concern that unilateral pedicle screw instrumentation may not offer adequate stabilization to prevent cage migration, especially without the use of an anterior column plate. Harris et al. have previously demonstrated in a human cadaveric model that unilateral pedicle screw fixation after transforaminal lumbar interbody fusion (TLIF) was associated with an increase of 182% in axial rotation compared to baseline (18). This instability was highlighted by Aoki et al. in a small case series of three patients who experienced cage migration post-TLIF approach with unilateral pedicle screw fixation (19). In this present study, six patients (out of 39 with ≥6 months of radiographic follow-up) experienced cage migration. Of these 6 patients, 2 did not achieve successful fusion. Methods to prevent cage migration may therefore play an important role in achieving successful arthrodesis, and a comparison of different posterior constructs in achieving successful fusion should be performed.
ASD is a term used to describe onset of new symptoms attributable to segments at levels adjacent to the level treated in the index surgical procedure (20). One of the hypotheses for the etiology of ASD involves increased biomechanical stress on adjacent spinal segments after fusion due to an increased lever arm from the fused segments (20). Another hypothesis involves destabilization of the spine from removal of bone and ligaments during decompression increasing wear on adjacent segments (21). Expanding on this hypothesis, several biomechanical studies have suggested open surgical approaches, with their increased paraspinal stripping and ligamentous destruction, may exacerbate this destabilization (22,23). The senior author of this present study hypothesized that unilateral pedicle screw fixation may provide adequate stabilization for an LLIF cage to achieve good fusion rates while minimizing destruction to the posterior elements. A review by Radcliff et al. reported an average incidence of ASD after spinal decompression and stabilization at 2–3% per year (21). Bae et al. reported an incidence of symptomatic ASD at 1.9% with average follow-up of 59 months (0.4% per year) in patients who received single level-fusion with percutaneous pedicle screw fixation. Ekman et al. reported an incidence of ASD as defined by radiographic criteria of 12.5% after 12.6 year follow-up (1.0% per year) in patients with interbody fusion and traditional bilateral pedicle screw fixation, with the majority (78%) of patients receiving single-level fusions (24). In the present study, the incidence of ASD was 5% after an average of 15 months (4% per year), which is higher than the reported rates of ASD from the prior studies. Interestingly, both patients with ASD in this present study developed ASD around 3 years after index procedure, so additional follow-up may present a more accurate yearly incidence of ASD following LLIF with unilateral pedicle screw fixation. The study of ASD has been challenging as there are no validated instruments to diagnose the condition (21). Therefore, different definitions of ASD may also affect the reported incidence of ASD. Efforts should be made to set a universally accepted definition of ASD.
Low grade subsidence occurred in almost all of our patients, with the cranial and caudal vertebral endplates having either Grade 0 or 1 subsidence in 100% of cases. A study by Marchi et al. on stand-alone LLIF cages reported Grade 0 or 1 subsidence in 77% of patients (10). One might consider defining low grade subsidence as an expected post-operative radiographic finding with no clinical significance in the majority of case. However, larger-scaled studies with uniform imaging are warranted to prove this hypothesis. Although subsidence has not been found to significantly affect clinical outcomes, there is a theoretical risk of nerve root compression and subsequent radiculopathy due to progressive loss of height of the intervertebral space (10).
The occurrence of neurological deficits following LLIF has been reported to be relatively common, with the majority of deficits resolving within 12 months, and may result from approach-related trauma to the lumbar plexus, either from traction or pressure-related ischemia and resultant neuropraxia (25,26). In this study, four patients developed neurological deficits of varying degrees during the hospital stay (9%). This is consistent with previous reports that have highlighted the potential injury to the lumbar plexus during the transpsoas approach (25,27,28).
This study has several limitations. It is a retrospective case series without any control group. Although the majority of patients were treated for spondylolisthesis with central canal stenosis (86%), a smaller group was treated for different indications. This may limit the generalizability of the study. Another limitation to this study was a relatively small sample size. Although statistical significance was achieved for many clinical parameters, there is a risk for false negatives due to inadequate power. Fusion was not assessed by gold standard CT imaging (29,30). However, fusion status was evaluated by a musculoskeletal fellowship-trained attending radiologist. Nevertheless, further studies with the utilization of CT imaging are warranted to further illuminate the radiographic outcome after LLIF supplemented with unilateral posterior instrumentation.
Conclusions
The results of the present study showed that patients who underwent unilateral pedicle screw instrumentation following LLIF may have significant improvement in clinical outcomes and favorable radiographic outcomes. Further investigation should involve a case-control study to compare bilateral to unilateral pedicle screw fixation for similar indications to evaluate clinical and radiographic outcomes. The use of unilateral pedicle screw fixation following LLIF may be a surgical option to further reduce surgical invasiveness.
Acknowledgements
We would like to acknowledge Jennifer Shue, M.S., C.C.R.C. for her support in this project.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board, and IRB number is 2014-097 at the Hospital for Special Surgery.
References
- Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine 2012;16:329-33.
- Marulanda GA, Nayak A, Murtagh R, et al. A cadaveric radiographic analysis on the effect of extreme lateral interbody fusion cage placement with supplementary internal fixation on indirect spine decompression. J Spinal Disord Tech 2014;27:263-70.
- Zhang H, Gao Q, Wang Y, et al. Clinical evaluation of indirect decompression treatments for degenerative adult idiopathic scoliosis. Arch Orthop Trauma Surg 2011;131:1639-47.
- Elowitz EH, Yanni DS, Chwajol M, et al. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg 2011;54:201-6.
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43.
- Rockoff SD, Sweet E, Bleustein J. The relative contribution of trabecular and cortical bone to the strength of human lumbar vertebrae. Calcif Tissue Res 1969;3:163-75.
- Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976) 2000;25:1077-84.
- Pimenta L, Turner AW, Dooley ZA, et al. Biomechanics of lateral interbody spacers: going wider for going stiffer. ScientificWorldJournal 2012;2012:381814.
- Isaacs RE, Hyde J, Goodrich JA, et al. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35:S322-30.
- Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8.
- Aichmair A, Lykissas MG, Girardi FP, et al. An institutional six-year trend analysis of the neurological outcome after lateral lumbar interbody fusion: a 6-year trend analysis of a single institution. Spine (Phila Pa 1976) 2013;38:E1483-90.
- Wiltse LL, Bateman JG, Hutchinson RH, et al. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg Am 1968;50:919-26.
- Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35:S331-7.
- Pimenta L, Marchi L, Oliveira L, et al. A prospective, randomized, controlled trial comparing radiographic and clinical outcomes between stand-alone lateral interbody lumbar fusion with either silicate calcium phosphate or rh-BMP2. J Neurol Surg A Cent Eur Neurosurg 2013;74:343-50.
- Cappuccino A, Cornwall GB, Turner AW, et al. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine (Phila Pa 1976) 2010;35:S361-7.
- Kepler CK, Sharma AK, Huang RC. Lateral transpsoas interbody fusion (LTIF) with plate fixation and unilateral pedicle screws: a preliminary report. J Spinal Disord Tech 2011;24:363-7.
- Le Huec JC, Liu M, Skalli W, et al. Lumbar lateral interbody cage with plate augmentation: in vitro biomechanical analysis. Eur Spine J 2002;11:130-6.
- Harris BM, Hilibrand AS, Savas PE, et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976) 2004;29:E65-70.
- Aoki Y, Yamagata M, Nakajima F, et al. Posterior migration of fusion cages in degenerative lumbar disease treated with transforaminal lumbar interbody fusion: a report of three patients. Spine (Phila Pa 1976) 2009;34:E54-8.
- Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 2004;4:190S-4S.
- Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J 2013;13:1339-49.
- Bresnahan L, Ogden AT, Natarajan RN, et al. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976) 2009;34:17-23.
- Kim DY, Lee SH, Chung SK, et al. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976) 2005;30:123-9.
- Ekman P, Möller H, Shalabi A, et al. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J 2009;18:1175-86.
- Lykissas MG, Cho W, Aichmair A, et al. Is There any Relation Between the Amount of Curve Correction and Postoperative Neurologic Deficit or Pain in Patients Undergoing Standalone Lateral Lumbar Interbody Fusion? Spine (Phila Pa 1976) 2013;38:1656-62.
- Pumberger M, Hughes AP, Huang RR, et al. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J 2012;21:1192-9.
- Taher F, Hughes AP, Lebl DR, et al. Contralateral motor deficits after lateral lumbar interbody fusion. Spine (Phila Pa 1976) 2013;38:1959-63.
- Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J 2014;14:749-58.
- Cook SD, Patron LP, Christakis PM, et al. Comparison of methods for determining the presence and extent of anterior lumbar interbody fusion. Spine (Phila Pa 1976) 2004;29:1118-23.
- Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol 2005;26:2057-66.


