Single anterior cervical discectomy and fusion (ACDF) using self- locking stand-alone polyetheretherketone (PEEK) cage: evaluation of pain and health-related quality of life
Introduction
Anterior decompression of the cervical spine was first introduced by Smith and Robinson and it was established by Cloward as a treatment of the cervical disc degeneration disease (DDD) (1-3). Since then, anterior cervical discectomy/decompression and fusion (ACDF) is used as the standard procedure for the treatment of single and multiple-level cervical DDD (4-6). ACDF is believed to have several advantages compared to discectomy alone (7). However, the scarcity of randomized studies is in accordance with the lack of an established gold standard method concerning ACDF (3). ACDF procedures with anterior cervical plating are thought to enhance stabilization with improvement in fusion, cervical alignment, implant subsidence and failure rates (8-10). Nevertheless, complications including increased dysphagia rates and implant-associated problems such as breakage, loosening of screws, screw penetration to endplate and fractures have been reported, especially in multilevel ACDF (11-14). Over the last decades, questions have also arisen in regards to the ideal technique (15) and the optimal material for ACDF (16).
After the use of autografts and allografts (17-19), the intervertebral cages, with or without plating, have become the most commonly used intervertebral implants (20,21). Cages were first used because of their theoretical ability to preserve disc height, restore lordosis and prevent implant collapse. The literature is abundant in studies comparing cages of different materials with autologous iliac bone implants as a control (3,22) and studies comparing anterior cervical discectomy with cage interbody fusion system (23). Cages that are filled with cancellous bone reduce donor pain, which is a common problem associated with iliac implants (24). There are several types of cages available for ACDF, including titanium, carbon fiber and polyetheretherketone (PEEK) cages. Complications from the use of ACDF using an interbody cage with anterior cervical plating have led some researchers to examine new systems to avoid these problems (25). Recently, a new type of self-locking stand-alone PEEK cage with no plates or screws has been designed. This cage has anti-migration teeth which are considered to be similar to a plate and screws, offering immediate stabilization. Studies have also shown that these cages can effectively restore cervical lordosis, and reduce plate-related complications (26).
C-Plus™ PEEK IBF System is an interbody fusion system designed to provide stability and anatomic restoration, facilitating fusion when used with autogenous bone graft. It is indicated for intervertebral body fusion of the spine in skeletally mature patients and intended for use at single level, from the C2-C3 disc to the C7-T1 disc, for the treatment of cervical DDD. The anatomic shape fits with the vertebral endplate, and the implant cage has a lordotic angle. Anti-backout teeth also act as additional stabilizers. The aim is to reduce instrumentation and the morbidity associated with traditional cervical anterior plating, while maintaining the benefits of interbody cages with anterior plating. We report our early experience on a prospectively collected series of patients treated with the C-Plus PEEK IBF System, at single level, and followed up to 12 months after surgery; indications, procedure steps, clinical outcomes and complications related to such new system are described. Although stand-alone cages have been frequently used for ACDF, the literature is still lacking studies evaluating both pain levels and quality of life regarding the use of new cages. The initial hypothesis stated that the parameters tested would present statistically important and gradual improvement postoperatively.
Methods
Patient population
All patients of our study were diagnosed with cervical DDD and they had filled all the indications for ACDF. All patients were referred to the same orthopaedic spine surgeon (SK) and all the procedures were performed at the same hospital, Interbalkan European Medical Center. Patients agreed to participate in the study and signed a fully informed written consent. The study has been approved by the hospital committee (Interbalkan European Medical Center, Thessaloniki, Greece) and the local ethics committee.
Inclusion criteria included: (I) adult individuals aged over 18 years old; (II) symptomatic cervical disc disease with cervical radiculopathy (including neck or arm pain and/or sensory/motor neurologic deficit on clinical examination), confirmed by magnetic resonance imaging (MRI) of the cervical spine, in compliance with clinical findings; (III) failure of at least 6 weeks of conservative treatment; (IV) single level cervical herniated disc. Exclusion criteria were: (I) multiple level cervical disc degeneration; (II) previous cervical spine surgery; (III) pre-operative dysphagia; (IV) allergy to cage materials; (V) active malignancy or infection or body mass index (BMI) ≥40; (VI) segmental instability; (VII) present vertebral fracture; (VIII) active rheumatic disease or metabolic bone disease affecting the cervical spine; (IX) pregnancy; (X) history of psychiatric disorder or recent substance abuse.
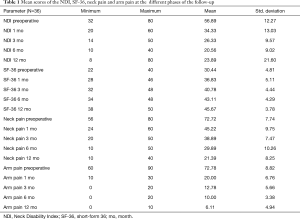
Between December 2015 and December 2016, 53 consecutive patients were selected to undergo ACDF for cervical disc herniation at levels from C3-C4 down to C6-C7 presenting with cervical radicular syndrome after failing conservative treatment for at least 6 weeks and corresponding findings on MRI. The implantation involved C-Plus self-locking stand-alone PEEK cages. Of those 53 patients, 39 (74%) patients had one level disease and were included in the study according to the criteria above. The rest 14 (26%) patients had multiple level disease. From the 39 patients, 37 were selected to participate in a one-year follow-up. During the follow-up period only one patient was lost (3%). The patient study sample consisted of 19 (53%) male and 17 (47%) female patients with ages ranging from 27 to 68 years (mean 49.6±7 years). Of the 36 enrolled patients 28 (78%) underwent ACDF at the C5-C6 level, which was the most common location. Cages with a 7-mm height were the most frequently implanted. Their health-related quality of life was evaluated by using the Neck and Arm pain as a form of visual analogue scale (VAS), as well as short-form 36 (SF-36) and Neck Disability Index (NDI). Patients were asked to complete the measurements immediately before surgery and postoperatively. They were estimated preoperatively and 1, 3, 6, and 12 months after the ACDF.
Surgical methods
A standard surgical technique for anterior cervical microdiscectomy was used (23). Stand-alone cervical anterior interbody fusion devices have already been used (27). All patients were monitored in terms of blood pressure, pulse rate, oxygen saturation and electrocardiographic signals. All patients were operated by the same orthopaedic surgeon (SK) using a standard, right-sided, Smith-Robinson technique. The surgical exposure involving access to the operating site and retraction of the tissues using minimal instrumentation. The trachea, esophagus, and coronary artery were retracted in order to clearly see the vertebral bodies and discs. The confirmation of level was done with the insertion of a marker into the disc and confirmation of the correct operative level utilizing a lateral radiograph. The discectomy and endplate preparation were performed using a curette and a rasp. This was followed by removal of the posterior longitudinal ligament, any disc material pressing the spinal cord and/or nerve roots, any osteophytes that were contacting the neural elements and cartilaginous endplates. The implant selection was performed with trial spacers. Webbed cage implant was used for more central support. The inner cavity of the cage was filled with autologous cancellous bone. Then the implant was inserted. Fluoroscopy was used intraoperatively to check the correct device’s placement.
Implant characteristics
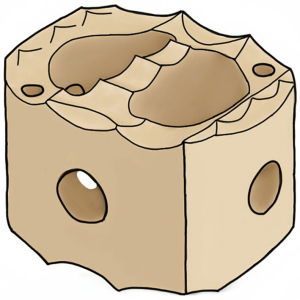
C-Plus PEEK IBF System has webbed and webless options available. Two vertical X-ray markers facilitate proper implant position and radiolucent material provides visibility of implant to observe progression of bony union. Anti-migration teeth resist implant migration and built-in lordosis of 7˚ allows anatomic restoration of patient’s curvature. Also, the convex inferior and superior surfaces optimize anatomic apposition of the implant with the concave endplates. A threaded inserter hole provides secures instrumentation and implant placement. The webbed implant offers more central support, while the webless implant offers ease of placement and continuity of bone graft material (Figure 1).
Data collection and outcome evaluation
Clinical outcomes were assessed preoperatively and at the follow-up phases. All patients were asked to complete questionnaires before surgery and at each follow-up examination. The self-reported measures used were the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36) (28), NDI (29), and VAS (25) scores. SF-36 scoring scale has 36 items. The second item is self-reported health changes and does not participate in scoring. The remaining 35 items constitute 8 dimensions, physiological function, physical function, bodily pain, general health, energy, social function, emotional function and mental health. The higher the total score of all these 8 dimensions, the better the quality of life survey. The NDI was used to measure the functional impact of the patients’ neck discomfort. It has 10 sections including pain intensity, personal care, lifting, reading, headaches, concentration, work, driving, sleeping, and recreation. The VAS was used to assess Neck and Arm Pain. After surgery, patients were assessed at 1, 3, 6 and 12 months. The incidence of dysphagia was recorded using the system defined by Bazaz (30). If respondents answered less than half of the number of entries then their questionnaires were considered invalid.
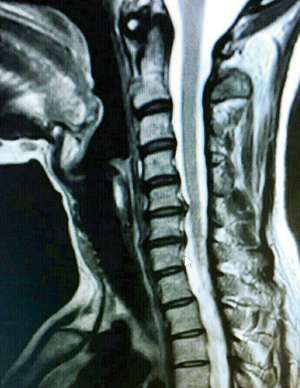
Pre-operative clinical evaluation was done by a team comprising of a neurologist, an orthopedic surgeon (SK) and a neurosurgeon. This included a pre-operative full neurological examination with thorough documentation of neck pain, radicular pain, and neurological deficits. Pre-operative radiological studies of the cervical spine included X-rays (anteroposterior, lateral, oblique and flexion/extension views), computed tomography (CT) and mainly MRI including T1 and T2-weighted sequences in transverse and sagittal planes (Figure 2). Postoperatively, static and dynamic X-rays were obtained at each follow-up visit to assess the position of the implanted devices and the fusion rate.
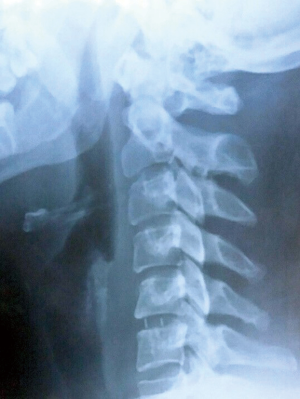
Assessment of fusion and subsidence was done with X-ray examinations on each phase of the follow up (Figure 3). A fusion was considered present if there was no motion of the fusion site on flexion-extension X-rays, no lucency on the fusion site and no individual osseous parts were observed. Subsidence was defined as a loss of height of more than 2 mm at the measured disc heights. Degenerative changes in the adjacent segments were evaluated on MRI at the final follow-up.
Data analysis
The statistical analysis of this study was performed with the statistical package SPSS, version 13.00 (SPSS Inc., Chicago, IL, USA). The P value <0.05 was determined as statistically significant difference level. Continuous variables were expressed as mean ± standard. We used Wilcoxon non-parametric test for related samples.
Results
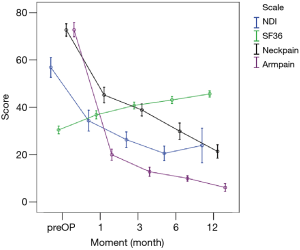
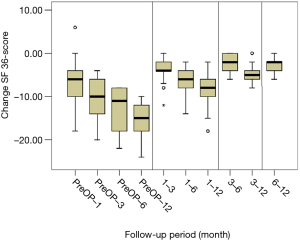
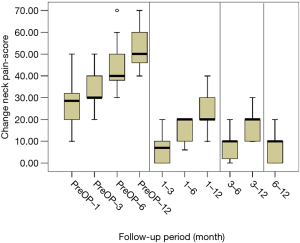
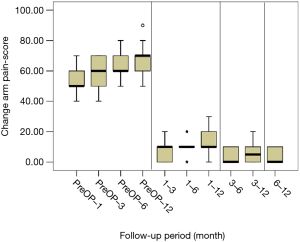
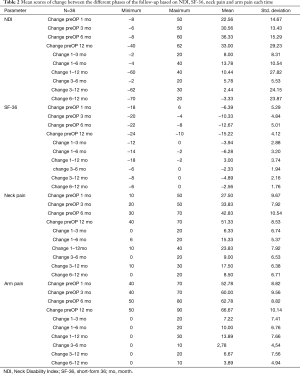
All scores present improvement postoperatively during the different phases of the follow-up (Figure 4, Table 1). SF-36, Neck Pain, as well as arm pain feature constant improvement during follow-up, with greatest scores presenting at 12 months with values of 45.67±3.78, 21.39±8.25 and 6.11±4.94 respectively. NDI presents its best score at 6 months postoperatively, 20.55±9.01. The change of mean scores between the different phases of the follow up for NDI, SF-36, neck pain and arm pain shows higher improvement in the first months postoperatively compared to the preoperative status and then gradual and constant amelioration up to the 12 months of the follow-up when compared with postoperative situation at 1 and 6 months (Figures 5-8, Table 2). As regards to the means between different phases, all results are statistically important (P<0.05), excluding improvement in NDI measured between 6 and 12 months (P=0.128) (Table S1).
Full table
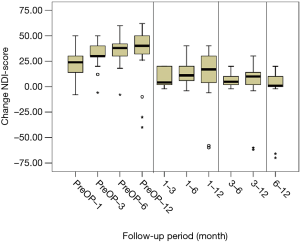
Full table

Full table
Operation time was estimated at 48.2±17.3 min and blood loss at 51.6±28.3 mL. Postoperative complications occurred in approximately 5% of the 36 patients. No device-related complications were encountered. There was no implant subsidence during follow-up and all patients had radiological evidence of fusion by 6 months postoperatively. However, the following complications occurred: one postoperative hematoma requiring surgical evacuation; one patient with mild dysphagia which was no longer present at the first follow up. Postoperatively, patients did not wear any cervical collar.
Discussion
ACDF is an effective and safe procedure for the surgical treatment of patients with cervical DDD. Cervical cages, the major aspect of the fusion process, were first introduced as a substitute for autologous iliac bone grafts avoiding autograft harvesting-related complications. Cages are characterized as cubical implants that are thought to restore physiological disc height and allow bone growth through the implant with osseous fusion (31). They accomplish internal fixation while, simultaneously, supplying structural support for the cancellous bone which is incorporated more quickly than an allograft (22). Furthermore, ACDF with anterior cervical plating results in a significantly higher incidence of fusion, alignment of the cervical spine, prevention of interbody cage dislocation or subsidence and decreased need for second surgery (8,10). However, complications including increased dysphagia rates, tracheoesophageal lesions and implant-associated problems are observed (20,32). These problems have led to the need for self-locking stand-alone cervical cages which do not require anterior cervical plating and are thought to have overcome the previous complications. The stand- alone cage has been used widely in clinical practice, and successful clinical results have been reported in more than one study (14,27). Until now, the literature is lacking of studies examining the quality of life after ACDF with the use of these cages. Our study examines the clinical outcomes as regards to pain levels and health-related quality of life of patients after ACDF using a unique self- locking stand-alone cage, without the use of plates or screws.
In the present study, SF-36, neck pain, arm pain and NDI presented generally significant improvement in the follow-up phases. The improvement was gradual and constant something that underlines the long-term effectiveness of the procedure. The fact that NDI at 6 months presents slightly better score than the one at 12 months, can be attributed to personal factors of the individuals which are of minor clinical importance. It is surprisingly important that pain levels as well as the quality of life show improvement confirming the initial hypothesis of the study. As regards to fusion, subsidence and complications, the results are surprisingly good and even better of the existing in the relevant literature. Only two minor complications occurred and the blood loss during surgery was mild. These outcomes are in accordance with the tendency of several recent studies which present the equality or even superiority of stand-alone cages over plating.
The latter is confirmed by various studies in the existing literature. Li et al. mentioned that the results were satisfactory and no significant differences in terms of improvement in the SF-36, VAS, NDI, Japanese Orthopedic Association scores, disc height, mean fusion time, fusion rate, adjacent segment degeneration, and restoration of cervical lordosis were observed after ACDF between stand- alone Fidji cervical cage and anterior cervical plate. Also, the cage group was associated with a lower risk of postoperative dysphagia, shorter operation time, less blood loss, less cost of index surgery, and relatively greater simplicity than the plate group (25). In a similar study, Azab et al. reported significant reduction in arm and neck pain, as well as, neck pain and disability scale maintenance over 12 months with no implant-associated complications and radiological fusion by 3 months in all patients treated with a zero-profile implant. None of the individuals had dysphagia after 3 months postoperatively (33). A zero-profile device is thought as a valuable alternative for ACDF with a low incidence of postoperative dysphagia and without segmental kyphotic change (34). Cho et al. found that the zero-profile anchored spacer has some advantage over stand-alone PEEK cage for maintaining segmental lordosis and lowering subsidence rate after single level ACDF (35). Barbagallo et al. mentioned that SF-36 and NDI showed a statistically significant improvement and mean arm pain VAS score deterioration on zero- profile cage-plate device. Fusion rate was estimated over 90% and dysphagia at 15.5% without any device-related complication (36). For pain and disability, NDI is said to be the most valid and responsive measure of improvement after surgery for neck and arm pain. However, for health-related quality of life, it is mentioned that only SF-12 PCS can accurately discriminate meaningful improvement after cervical surgery and is found to be most valid and responsive (37).
The stand-alone cage may be associated with a significantly lower estimated blood loss, which may not be clinically relevant, however perioperative outcomes, complications, reoperation rates, narcotics consumption in the immediate postoperative period, and total costs may be similar to anterior plating (38). In a prospective randomized study, the stand-alone anchored PEEK cage was confirmed as a valid alternative to plating in ACDF with a low rate of adjacent- level ossification (39). ACDF with stand-alone spacers has resulted in similar clinical and radiologic outcomes as compared with plate and spacers and may help minimize postoperative dysphonia (40). As regards to three-level cervical degenerative spondylopathy, ACDF using self-locking stand-alone cages have shown similar clinical results as compared to ACDF using cages and plate fixation (41). Also, cervical lordosis is considered to play an important role in the maintenance of surgical results and sagittal malalignment is thought to increase the incidence of failure of internal fixation and disease in adjacent segments (42). It has influence on cervical instability, postoperative pain, deterioration of neurological deficit, and functional recovery. Depending on that, the new stand- alone cervical cages have built-in lordosis for anatomic restoration.
On the other hand, Fraser et al. reported a significantly increased rate of fusion if an additional anterior plate had been used for either single level or multilevel ACDF procedures (8). Song et al. compared two groups of patients treated with ACDF, respectively, with cage alone and with cage and plate and they found that the use of a plate was associated with a better sagittal alignment, higher fusion rate and lower cage subsidence and complication rates (9). However, no significant differences in clinical outcomes were observed between the two groups. Pitzen et al. found that when a dynamic plate is used the fusion process is faster and the implant-related complications are lower, moreover the advantages are counterbalanced by a greater loss of segmental lordosis at 2-year follow-up than that observed in patients treated with a rigid plate (10). In addition, stand-alone cage has showed higher incidence rate than plate-assisted cervical fusion in segmental subsidence and cervical kyphosis (43).
The use of cervical anterior plates has been associated with various intra-operative and postoperative complications. Examples of implant-associated complications include screw loosening, screw breakage and plate breakage. According to Fountas et al. (32), postoperative soft-tissue edema, esophageal injury, postoperative hematoma, and adhesive formations around plates might be possible explanations for dysphagia-related symptoms. Persistent long-term dysphagia can be caused by adhesions between posterior esophagus and the plate. Hilibrand et al. reported that approximately one out of four of patients who underwent single-level ACDF developed adjacent level disease within 10 years (44).
The C-Plus PEEK cervical cage can be completely contained in the decompressed intervertebral space, with minimal tissue damage while avoiding the mechanical stimulus to the esophagus and other pre-vertebral soft tissues, something which explains the lower postoperative dysphagia rates and preserves the normal anatomy of the region. It also minimizes the risk of ASD while it remains within the index disc space, without reaching the adjacent segments. It is important to mention that the instrumentation is the least feasible without the use of any plate or screw. This cage system seems to be a valid alternative to anterior cervical plating in patients undergoing ACDF and it is characterized by a very low incidence of postoperative dysphagia and no implant-related complications over a minimum follow up period of 1 year.
This study is limited by the relatively small number of patients. However, the selected individuals are carefully chosen. In addition, the mean follow-up period was too short to evaluate the long-term efficacy of the new stand-alone cervical cage. Nevertheless, we feel that this study provides useful information regarding the surgical treatment of cervical DDD. Further large-scale, prospective, randomized studies with long-term follow-up periods are needed to overcome these limitations and definitively determine whether this new cervical cage has advantages over the stand-alone cages with a titanium plate for ACDF. There were some limitations in our study, such as the difficulty in the assessment of bone fusion with plain radiographs, and non-feasibility of assessing every patient with CT scan. The shorter operation time and less blood loss of ACDF using self- locking stand-alone cage indicates less traction time and less damage of prevertebral soft tissues during surgery, which may have contributed to the relatively low rate of dysphagia.
Conclusions
ACDF using self-locking stand-alone cages can help achieve similar or even better clinical results as that achieved by ACDF using cage and plate fixation for the treatment of cervical DDD. In summary, the clinical and radiological outcome of the new cervical cage for the surgical treatment of single level cervical DDD was satisfactory after a minimum 1-year follow-up in terms of improvement in the SF-36, VAS, NDI and pain levels. Overall, the results show that the C-Plus cervical cage for ACDF is an effective, reliable, and safe alternate to the conventional method for the treatment of cervical DDD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study has been approved by the hospital committee (Interbalkan European Medical Center, Thessaloniki, Greece) and the local ethics committee. Patients agreed to participate in the study and signed a fully informed written consent.
References
- Cloward RB. The anterior approach for removal of ruptured cervical disks. 1958. J Neurosurg Spine 2007;6:496-511. [Crossref] [PubMed]
- Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp 1955;96:223-4.
- Thomé C, Leheta O, Krauss J, et al. A prospective randomized comparison of rectangular titanium cage fusion and iliac crest autograft fusion in patients undergoing anterior cervical discectomy. J Neurosurg Spine 2006;4:1-9. [Crossref] [PubMed]
- Korinth MC. Treatment of cervical degenerative disc disease: current status and trends. Zentralbl Neurochir 2008;69:113-24. [Crossref] [PubMed]
- Matz PG, Ryken TC, Groff MW, et al. Joint Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons and Congress of Neurological Surgeons Technique for anterior cervical decompression for radiculopathy. J. Neurosurg Spine 2009;11:183-97. [Crossref] [PubMed]
- Mummaneni PV, Kaiser MG, Matz PG, et al. Joint Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons and Congress of Neurological Surgeons Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine 2009;11:130-41. [Crossref] [PubMed]
- Thorell W, Cooper J, Hellbusch L, et al. The long-term clinical outcome of patients undergoing anterior cervical discectomy with and without intervertebral bone graft place ment. Neurosurgery 1998;43:268-73. [Crossref] [PubMed]
- Fraser JF, Hartl R. Anterior approaches to fusion of the cervical spine: A metaanalysis of fusion rates. J Neurosurg Spine 2007;6:298-303. [Crossref] [PubMed]
- Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976) 2009;34:2886-92. [Crossref] [PubMed]
- Pitzen TR, Chrobok J, Stulik J, et al. Implant complications, fusion, loss of lordosis, and outcome after anterior cervical plating with dynamic or rigid plates: two year result of a multicentric, randomized, controlled study. Spine (Phila Pa 1976) 2009;34:641-6. [Crossref] [PubMed]
- Ning X, Wen Y, Xiao-Jian Y, et al. Anterior cervical locking plate-related complications; prevention and treatment recommendations. Int Orthop 2008;32:649-55. [Crossref] [PubMed]
- Kasimatis GB, Panagiotopoulos E, Gliatis J, et al. Complications of anterior surgery in cervical spine trauma: an overview. Clin Neurol Neurosurg 2009;111:18-27. [Crossref] [PubMed]
- Riley LH, Skolasky RL, Albert TJ, et al. Dysphagia after anterior cervical decompression and fusion. Spine 2005;30:2564-9. [Crossref] [PubMed]
- Scholz M, Schnake KJ, Pingel A, et al. A new zero- profile implant for stand-alone anterior cervical interbody fusion. Clin Orthop Relat Res 2011;469:666-73. [Crossref] [PubMed]
- van Limbeek J, Jacobs WC, Anderson PG, et al. A systematic literature review to identify the best method for a single level anterior cer vical interbody fusion. Eur Spine J 2000;9:129-36. [Crossref] [PubMed]
- Wigfield CC, Nelson RJ. Non autologous interbody fusion materials in cervical spine surgery: How strong is the evidence to justify their use? Spine 2001;26:687-94. [Crossref] [PubMed]
- Epstein NE. Iliac crest autograft versus alternative constructs for anterior cervical spine surgery: pros, cons, and costs. Surg Neurol Int 2012;3:S143-56. [Crossref] [PubMed]
- Jacobs W, Willems PC, Kruyt M, et al. Systematic review of anterior interbody fusion techniques for single- and doublelevel cervical degenerative disc disease. Spine (Phila Pa 1976) 2011;36:E950-60. [Crossref] [PubMed]
- Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine (Phila Pa 1976) 1991;16:S561-4. [Crossref] [PubMed]
- Niu CC, Liao JC, Chen WJ, et al. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech 2010;23:310-6. [Crossref] [PubMed]
- Ryken TC, Heary RF, Matz PG, et al. Joint Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons and Congress of Neurological Surgeons Techniques for cervical interbody grafting. J Neurosurg Spine 2009;11:203-20. [Crossref] [PubMed]
- Hacker RJ, Cauthen J, Gilbert T, et al. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine (Phila Pa 1976) 2000;25:2646-54; discussion 2655. [Crossref] [PubMed]
- Bartolomei J, Sonntag V. Anterior approach including cervical corpectomy. In: Winn HR, editor. Youmans’ Neurological Surgery. Philadelphia: Saunders, 2004:4431-45.
- Vavruch L, Hedlund R, Javid D, et al. A prospective randomized comparison between the cloward procedure and a carbon fiber cage in the cervical spine: a clinical and radiologic study. Spine (Phila Pa 1976) 2002;27:1694-701. [Crossref] [PubMed]
- Li Z, Zhao Y, Tang J, et al. A comparison of a new zero-profile, stand-alone Fidji cervical cage and anterior cervical plate for single and multilevel ACDF: a minimum 2-year follow-up study. Eur Spine J 2017;26:1129-39. [Crossref] [PubMed]
- Zhou J, Li X, Dong J, et al. Three-level anterior cervical discectomy and fusion with selflocking stand-alone polyetheretherketone cages. J Clin Neurosci 2011;18:1505-9. [Crossref] [PubMed]
- Scholz M, Reyes PM, Schleicher P, et al. A new stand-alone cervical anterior interbody fusion device. Spine 2009;34:156-60. [Crossref] [PubMed]
- King JT Jr, Roberts MS. Validity and reliability of the Short Form-36 in cervical spondylotic myelopathy. J Neurosurg 2002;97:180-5. [PubMed]
- Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409-15. [PubMed]
- Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: A prospective study. Spine 2002;27:2453-8. [Crossref] [PubMed]
- Assietti R, Beretta F, Arienta C. Two-level anterior cervical discectomy and cage-assisted fusion without plates Neurosurg Focus 2002;15;12:E3.
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Azab W, Abdel-Razek M, Ali A, et al. Outcome evaluation of a zero-profile implant for anterior cervical diskectomy with fusion. Turk Neurosurg 2012;22:611-7. [PubMed]
- Shin JS, Oh SH, Cho PG. Surgical Outcome of a Zero-profile Device Comparing with Stand-alone Cage and Anterior Cervical Plate with Iliac Bone Graft in the Anterior Cervical Discectomy and Fusion. Korean J Spine 2014;11:169-77. [Crossref] [PubMed]
- Cho HJ, Hur JW, Lee JB, et al. Cervical Stand-Alone Polyetheretherketone Cage versus Zero-Profile Anchored Spacer in Single- Level Anterior Cervical Discectomy and Fusion: Minimum 2-Year Assessment of Radiographic and Clinical Outcome. J Korean Neurosurg Soc 2015;58:119-24. [Crossref] [PubMed]
- Barbagallo GM, Romano D, Certo F, et al. Zero-P: a new zero-profile cage-plate device for single and multilevel ACDF. A single Institution series with four years maximum follow-up and review of the literature on zero-profile devices. Eur Spine J 2013;22:S868-78. [Crossref] [PubMed]
- Godil SS, Parker SL, Zuckerman SL, et al. Accurately measuring the quality and effectiveness of cervical spine surgery in registry efforts: determining the most valid and responsive instruments. Spine J 2015;15:1203-9. [Crossref] [PubMed]
- Tabaraee E, Ahn J, Bohl DD, et al. Comparison of Surgical Outcomes, Narcotics Utilization, and Costs After an Anterior Cervical Discectomy and Fusion: Stand-alone Cage Versus Anterior Plating. Clin Spine Surg 2016. [Epub ahead of print].
- Nemoto O, Kitada A, Naitou S, et al. Stand- alone anchored cage versus cage with plating for single-level anterior cervical discectomy and fusion: a prospective, randomized, controlled study with a 2-year follow-up. Eur J Orthop Surg Traumatol 2015;25 Suppl 1:S127-34. [Crossref] [PubMed]
- Panchal RR, Kim KD, Eastlack R, et al. A Clinical Comparison of Anterior Cervical Plates versus Stand-Alone Intervertebral Fusion Devices for Single-Level Anterior Cervical Discectomy and Fusion Procedures. World Neurosurg 2017;99:630-7. [Crossref] [PubMed]
- Chen Y, Lu G, Wang B, et al. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking stand-alone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: a retrospective study with 2-year follow-up. Eur Spine J 2016;25:2255-62. [Crossref] [PubMed]
- Harrison DD, Harrison DE, Janik TJ, et al. Modeling of the sagittal cervical spine as a method to discriminate hypolordosis: results of elliptical and circular modeling in 72 asymptomatic subjects, 52 acute neck pain subjects, and 70 chronic neck pain subjects. Spine (Phila Pa 1976) 2004;29:2485-92. [Crossref] [PubMed]
- Han SY, Kim HW, Lee CY, et al. Stand-Alone Cages for Anterior Cervical Fusion: Are There No Problems? Korean J Spine 2016;13:13-9. [Crossref] [PubMed]
- Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 2004;4:190S-4S. [Crossref] [PubMed]