
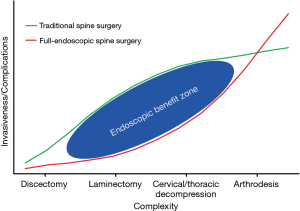
The benefit zone of full-endoscopic spine surgery
Introduction
Over the past 50 years, the field of spine surgery has seen an increasing emphasis in techniques designed to minimize approach-related tissue trauma and, hence, reduce post-operative pain and disability. The term “minimally invasive” represents a shift in the philosophical framework of spine surgery and echoes the fundamental Hippocratic aphorism “do no harm”. The actualization of this paradigm shift is predicated upon a precision diagnosis and a targeted treatment which takes into account patient comorbidities and short-term and long-term expectations. Rather than focusing on the length of an incision, the “invasiveness” or collateral damage that is associated with a surgical procedure, must be taken into account when selecting the optimal treatment.
The emphasis on decreasing muscle crush injuries during prolonged retraction, avoiding soft tissue stripping and muscular denervation, minimizing bony resection to prevent iatrogenic instability and utilizing a surgical corridor that allows targeted access to spinal pathology are all principles which every surgeon should employ when treating patients. Full-endoscopic spine surgery represents the evolution of minimally-invasive surgical access to spinal pathology. Although the first spinal endoscopic procedures were performed in the early 1980’s in the United States (1), there has been a steady and persistent increase in popularity, particularly in Europe and Asia. Multiple studies (2-7) have demonstrated endoscopic lumbar decompression provides equivalent outcomes to microsurgical or tubular techniques with shorter hospital stay (8) and less collateral tissue injury (9,10) and less systemic stress for the patient (11,12). With continued evolution in surgical techniques, technological innovations, and approaches for the cervical (13,14) and thoracic spine (15,16), endoscopic techniques have been gaining broader appeal.
Endoscopic spine surgical techniques represent another tool in the surgical armamentarium of the spine surgeon to treat spinal pathology using these principles. Rather than being touted as a panacea for all spinal pathology, full-endoscopic spine surgery offers distinct advantages which vary based on the overall health status of the patient and the technical complexity of the planned procedure. As with all new technologies and procedures, rigorous scrutiny of potential advantages and limitations are critical in delineating the true benefit of wider adoption and implementation. We present a brief review of the history of endoscopic spine surgery, an overview of current techniques and review current outcomes of endoscopic spine surgical procedures in the context of an invasiveness/complexity index to elucidate the benefit zone of these newer techniques.
Brief history of endoscopic spine surgery
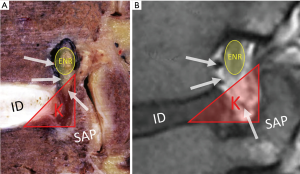
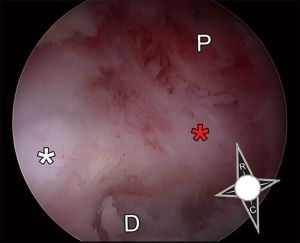
While the first rudimentary endoscope for cystoscopy was created in the early 19th century (17), a century of technological advancements led to adoption of endoscopic techniques in almost all of surgical subspecialties (18). The 20th century witnessed the rise of arthroscopic surgery, which has undoubtedly, changed the field of orthopaedic surgery. Although arthroscopy continued to evolve with continued technological advancements and gained widespread appeal in orthopaedic surgery, endoscopic techniques were largely abandoned in spine surgery due to the morbidity associated with insertion of a large-bore endoscope into the dural cavity (19). It was not until the early 1970s when endoscopic spine surgery gained a renewed interest. Ironically, the precursor to modern day endoscopic spine techniques was heralded by a “blind” nucleotomy or discectomy. Borrowing from principles from percutaneous biopsy of vertebral body lesions (20-22), a technique for fluoroscopic-guided percutaneous non-visualized discectomy under local anesthesia was described by Parvis Kambin in 1973 (23) and Hijikata in 1975 (24). Using specialized cannulas and instruments without endoscopic visualization, these techniques represented “intra-discal” indirect decompression procedures to address posterolateral disc herniations via removal of the posterior one third of the nucleus pulposus. Using these techniques Kambin et al. reported their results from a prospective series of 100 patients with 1–6 years follow up with symptomatic lumbar herniations with unremitting radicular pain. They reported an 87% success rate based on a modified MacNab criteria, patient interview and examination and questionnaire (25). Although it is difficult to decipher the meaning of these results as the percutaneous discectomy cohort had narrow indications and was not matched to any controls, these results were nonetheless promising and led to further interest in endoscopic techniques. Early endoscopic spine surgery revolved the extra-foraminal and neural foraminal area, resulting in extensive anatomical investigation. Kambin conducted numerous cadaveric studies to describe the boundaries of a safe working zone for posterolateral access to the disc space (1,26). He defined Kambin’s triangle, a theoretical triangle for safe access into the disc over the posterolateral disc: the hypotenuse is the exiting nerve root, the base (width) is the superior border of the caudal vertebra, and the height is the dura/traversing nerve root. The triangle is loosely covered by adipose tissue and small superficial veins as well as suspensory ligaments tethering the neural structures (Figure 1). As surgeon’s gained comfort with this anatomical trajectory, the cannulas utilized for performing a discectomy increased in size to allow larger instruments to be passed through (27,28) and principles of arthroscopy were utilized to provide visualization of the procedure (29).
Transforaminal technique
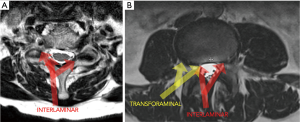
While the early procedures could be described as extra-foraminal and disc-based, the transition of the endoscope into the foramen marked the beginning of the present-day transforaminal endoscopic discectomy (30). In the late 1990s, Yeung designed the YESS endoscope, a 510k FDA approved multi-channel fluid integrated working channel rigid endoscope, to perform transforaminal endoscopic discectomy (31). Yeung’s technique was derived from Kambin’s arthroscopic discectomy technique (32) and Schreiber and Suezawa’s use of injecting indigo carmine into the disc space to stain the abnormal nucleus pulposus and annular fissures (33,34). The technique was primarily an intra-discal or “inside-out” procedure, however, Yeung described the use of lasers and other bone cutting instruments to perform a foraminoplasty to expand the foramen and improve visualization post-discectomy (31). By the mid 2000s, Schubert and Hoogland (35) described their technique for transforaminal endoscopic removal of a sequestered disc fragment using reamers to first expand the foraminal window by removing the ventral portion of the superior articular process. This was a philosophical shift returning to traditional spine surgery, in which visualization was required prior to performing a discectomy. This marked the beginning of an “outside-in” approach, which diverged from the earlier transforaminal techniques which were primarily intra-discal in nature. We believe the “outside in” philosophy represents a major evolution of the transforaminal technique and allows for the use of bony principal anatomical landmarks similar to traditional spine surgery. Transforaminal techniques can currently be performed in the lumbar and thoracic spine (Figure 2).
Interlaminar technique
While transforaminal endoscopic surgery was slowly evolving, the initial learning curve and lack of access to expert training resulted in slow adoption. Concurrently, the development of the tubular retractor system by Destandau (36) and Foley (37) in the late 1990’s, heralded a new era of minimally invasive techniques utilizing an interlaminar window. Although these techniques initially were endoscopically-assisted and resulted in other interlaminar endoscopic systems to be developed (38), the use of the microscope soon supplanted the endoscope among most spine surgeons. However, with advancements in endoscopic technology and techniques (39), endoscopic interlaminar approaches have been regaining popularity. Current interlaminar endoscopic techniques mirror those of tubular techniques with the added advantage of improved visualization and more targeted placement due to the maneuverability of a narrow endoscope and the ability to manipulate the field of view with optical rotation of the endoscope. For example, endoscopic unilateral laminotomy for bilateral decompression (ULBD) for lumbar spinal stenosis allows for excellent ipsilateral facet joint preservation given off-angle visualization and the ability to tilt the small diameter endoscope out into the lateral recess (Figure 3). These features allow for generous decompression of the nerve root beyond the caudal index level pedicle when performing a posterior endoscopic cervical foraminotomy. Interlaminar techniques can currently be performed in the cervical, thoracic and lumbar spine (Figure 2).
The surgical invasiveness and complexity index
The surgical invasiveness and complexity index is a qualitative graphical representation which plots the perceived complexity and invasiveness of spine surgical procedures which can be accomplished via open, minimally-invasive tubular or endoscopic methods (Figure 4). The complexity and invasiveness of a procedure are based on best available data regarding expected outcomes and the number and magnitude of potential complications. The least invasive and complex surgery is a lumbar microdiscectomy; a lumbar laminectomy or laminotomy for lumbar spinal stenosis is low to moderate invasiveness/complexity depending on the number of levels requiring decompression and the presence of co-existing structural pathology (deformity or spondylolisthesis). Moderate to higher complexity procedures include any decompression of the cervical or thoracic spine due to the proximity to the spinal cord and potential need for ventral decompression. Finally, higher complexity procedures involve revision surgery and previous arthrodesis as these procedures are known to have higher complications and require more surgical exposure to address spinal pathology. When determining if a particular patient will benefit from endoscopic spine surgery we attempt to estimate the reduction of invasiveness compared to a traditional open surgery while making sure to achieve the same surgical goals.
Low complexity procedures
Lumbar microdiscectomy
Full-endoscopic spine surgery evolved from a percutaneous discectomy, hence, there is an abundance of literature supporting the use of endoscopic surgery for performing lumbar microdiscectomy. There are numerous randomized controlled and prospective studies that investigate clinical outcomes following full endoscopic) microdiscectomy. Ruetten et al. (40) conducted a prospective controlled study of 200 patients who were randomized to either full-endoscopic discectomy (transforaminal or interlaminar) or open microsurgical discectomy with 2-year follow-up. Both groups experienced similar improvements in pain and function, however, a statistically significant number of patients in the microsurgical group experienced greater back pain post-operatively. There were no significant differences in reoperation rates between the two groups, however, the endoscopic cohort was found to have statistically significant less complications, less post-operative pain medication requirements and less postoperative work disability. Similarly, Gibson et al. (2) conducted a prospective randomized controlled study of 140 patients who underwent endoscopic transforaminal discectomies or open microsurgical discectomy with 2-year follow-up available on 123 patients. While both cohorts noted significant improvement from baseline, VAS leg pain scores at 2 years were significantly less following endoscopic discectomy (1.9±2.6) when compared to microsurgical discectomy (3.5±3.1, P=0.002). There was no significant difference in reoperation and complication rates between the cohorts, however, the endoscopic group was found to have a significantly shorter length of hospital stay.
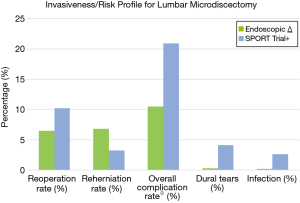
Two recent meta-analysis (41,42) suggest equivalent or superior patient reported outcome measures and shorter hospital stay with no difference in reoperation, recurrence or complication rates. Table 1 lists an aggregate of 540 patients who underwent endoscopic discectomy from 5 randomized controlled and prospective studies with 1- to 2-year follow-up; the average reoperation rate is 6.5% and the overall complication rate (including all peri-operative complications and reoperations) is 10.5%. The incidence of incidental durotomy and infection were both 0.3%. When looking at data from the randomized controlled Spine Outcomes Research Trial (SPORT) for microsurgical discectomy versus non-operative treatment of lumbar disc herniation, the average reoperation rate was 7.4% at 1 year and 10.2% at 2 years (43). This is similar to endoscopic discectomy, however, the SPORT trial reported an overall 20.9% complication rate (data includes all reoperations) including a 4.1% incidence of incidental durotomies and a 1.6% infection rate. The SPORT trial reported a recurrent re-herniation rate of 3.29%, which is lower than the average rate of 6.8% in the endoscopic aggregate cohort (Figure 5). Although the endoscopic recurrence rate may seem marginally higher, it is well within the range reported within the literature (44,45). Reherniation following discectomy has been shown to vary with patient-specific factors (46,47) as well as technical factors such as the aggressiveness of the degree of disc removal (48,49). Moreover, there is variability in endoscopic technique, where some surgeons may spend more time widening the foraminal window to visualize more of the ventral epidural space. Regardless, benefits of endoscopic techniques lie more so in the context of overall surgical morbidity. Interestingly, a recent randomized controlled trial found that transforaminal endoscopic discectomy was found to have better outcomes for far-lateral disc herniations, while an endoscopic-assisted interlaminar technique was found to have better outcomes for median disc herniations (50). A recent multi-institutional cohort study of 553 consecutive full endoscopic cases including 377 endoscopic discectomies reported an overall complication rate of 2.4% (51). Although endoscopic discectomy offers a faster recovery with a highly favorable risk profile, overall outcomes are similar to open techniques. Many surgeons may not tolerate the learning curve of a new procedure for marginal benefits. For the spectrum of simple, low complexity procedures, the benefits of endoscopic spine surgery exist but remain incremental.

Full table
Moderate complexity procedures
Lumbar laminectomy
The surgical treatment of lumbar spinal stenosis with or without structural abnormalities (deformity, spondylolisthesis) represents low to moderate complexity procedures. Open laminectomy for spinal stenosis has been shown to be a safe and cost-effective procedure, with superior outcomes when compared to non-surgical management (52-58). However, performing adequate bony decompression of neural elements may cause long-term segmental spinal instability leading to recrudescence of symptoms or subsequent need for fusion (59,60). This is particularly important in patients with coexisting deformity or spondylolisthesis given early experiences with poor outcomes (61,62) and curve progression when these patients undergo traditional open laminectomy (63,64). There have been two recent randomized controlled trials comparing decompression versus decompression and fusion for lumbar spinal stenosis with or without spondylolisthesis with conflicting conclusions, the Swedish Spinal Stenosis Study and the SLIP trial (64,65). Försth et al. reported on a large heterogeneous group of 247 patients with one to two level spinal stenosis with or without coexisting spondylolisthesis that were randomized to open laminectomy versus fusion. The authors reported that the addition of fusion did not result in any improvement in outcomes (ODI) at both 2 and 5 years, even after adjusting for spondylolisthesis; with a mean follow-up of 6.5 years, there was a 22% and 21% reoperation rate in the fusion and laminectomy groups, respectively. Ghogawala et al. reported on a smaller more homogenous group of 66 patients with one level spinal stenosis with associated grade I stable spondylolisthesis that were randomized to open laminectomy versus posterolateral fusion. The authors reported that the fusion group had significant improvements in some patient reported outcomes (SF-36 PCS), but no significant improvements in ODI at 2- and 4-year follow-up; when adjusting for preoperative differences in disability and spondylolisthesis between the groups, there was no significant difference in all outcome scores. However, there was a 14% cumulative reoperation rate in the fusion group and a 34% reoperation rate in the decompression group (P=0.05).
When looking at both of these excellent trials, there are several salient points which warrant closer examination. The rate of complications and reoperation rates in the open laminectomy groups in these trials is not trivial. Försth et al. reported a 21% reoperation rate, an 11% rate of dural tears, a 4% infection rate and 4% rate of other post-operative medical complications. Ghogawala reported a 34% reoperation rate and a 6% rate of major complications. When looking at the SPORT trial for the treatment of patients with spinal stenosis without spondylolisthesis (62), the rate of re-operation at 4 years was 13%, the rate of dural tears was 10%, the rate of infection was 3%, the rate of post-operative transfusion was 5% and the rate of all perioperative complications (not including reoperation) was 18%. Despite the lower complexity of an open laminectomy, the morbidity associated with this procedure is not negligible.
Minimally invasive techniques such as tubular unilateral laminotomy for bilateral decompression (ULBD) have been shown to provide comparable outcomes with open techniques with less overall morbidity (66,67). In a prospective randomized controlled trial of 79 patients undergoing tubular ULBD versus open laminectomy for spinal stenosis with average 3-year follow-up, the minimally invasive alternative was shown to have overall equivalent patient reported outcomes to open laminectomy with shorter hospital stay, less blood loss and less opioid pain requirements (68). A recent 2017 prospective study with 2-year follow-up compared outcomes in 207 patients with spinal stenosis with or without co-existing scoliosis (mean cobb angle 14º) following tubular-based ULBD (69) and found significant increases in functional outcomes in all patients with only an 8% reoperation rate; this contrasts to reported reoperation rates of 25–37% (70,71) in patients with significant deformity undergoing open laminectomy. Unfortunately, the alternatives to an isolated decompression in this patient population are short segment and long fusions, which not only have higher rates of complications (72) but also have high rates of adjacent segment reoperation (71), exposing some patients to a lifetime of fusion extensions.
Endoscopic spine surgery represents the evolution of tubular minimally invasive techniques; the advantage of full-endoscopic surgery are a small working corridor with minimal irritation of the paraspinal muscles, constant irrigation which provides a clear operative field and gentle general retraction of the thecal sac and nerve roots as well as an angled view resulting in the ability to effectively undercut the fact joint (Figure 3). Minamide et al. recently reported on 242 patients with spinal stenosis and degenerative spondylolisthesis who underwent microendoscopic (endoscopic-assisted tubular) ULBD with a mean 4.6-year follow-up. They reported excellent/good recovery of JOA score in approximately 70% of patients and a reoperation rate of 7.8%, with only 5% of patients requiring fusion at last follow-up. Interestingly, based on radiographic analysis, the rate of progressive instability was 7.8% with restabilization of the spine in 35% of patients with preoperative instability. Notably, there was an overall complication rate of 4.5% which included a 1.2% rate of dural tears, a 1.7% rate of epidural hematomas and a 0.4% infection rate.
At our institution, we routinely employ endoscopic techniques to treat lumbar spinal stenosis with or without coexisting degenerative deformity or spondylolisthesis. In our series of 108 patients who underwent endoscopic decompression for spinal stenosis there were 3 patients (3%) who underwent reoperation with 2-year follow-up. One patient developed adjacent segment disease requiring ultimate fusion, one patient developed a synovial cyst at the index level and one patient had a disc herniation at the index level. Notably, there was only 1 dural tear (0.9%) and no infections. A comparison of the invasiveness and risk profile for endoscopic decompression versus open laminectomy for spinal stenosis can be seen in Figure 6. Endoscopic spine surgery not only lessens the morbidity associated with open decompression, but it may also decrease the risk of destabilization, hence, allowing for a broader application to address more advanced structural pathology. We believe that for low to moderate complexity procedures, endoscopic decompression offers substantial benefits over a traditional open laminectomy.

High complexity procedures
Cervical and thoracic decompression
As the use of endoscopic surgical techniques continues to expand to treat a wider range of pathology, the application of endoscopy has extended to treat compressive pathology in the cervical and thoracic spine. The treatment of symptomatic thoracic disc herniation with ventral compression of the spinal cord represents a challenging problem for many spine surgeons. Current accepted approaches include posterolateral costotransversectomy (73), posterior transpedicular (74), lateral extracavitary (75), transthoracic (76) and video-assisted thoracoscopic surgery (VATS) (77). Many of these traditional approaches require either significant soft tissue stripping or extensive bone resection or require entrance into the thoracic and pleural cavities with its associated morbidity. Fessler et al. reviewed reported complications following various approaches to address thoracic disc herniations and found that in 242 patients from 19 reports, approximately 26% of procedures were associated with significant morbidity and mortality (78). These procedures, whether retropleural or intrapleural, can result in significant perioperative morbidity secondary to pain, difficult ventilation requiring prolonged ICU stay, shoulder girdle dysfunction, and wound healing problems (79,80). Even with more minimally-invasive thoracoscopic methods such as VATS, there was a 21% rate of complications (77). The endoscopic transforaminal approach offers the ability to safely access thoracic disc herniations with minimal bony and soft tissue disruption while avoiding entrance into the thoracic cavity. Choi et al. reported on 14 patients with a soft thoracic disc herniation who underwent endoscopic transforaminal thoracic discectomy with average 5-year follow-up (81). There were significant improvements in VAS and ODI from baseline and no surgery-related complications were observed and no conversion to an open procedure was required. Similarly, Nie et al. reported 77% excellent/good results for 13 patients who underwent endoscopic transforaminal thoracic discectomy with average 17-month follow-up (82). They reported a 0.08% complication rate with one dural tear treated successfully with a blood patch. Although evidence for endoscopic techniques in the thoracic spine are currently limited to case series and will require more investigation, the current available data remains promising.
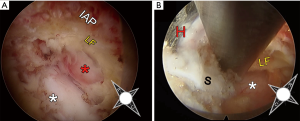
Currently applications of endoscopic spine surgery to treat cervical pathology include posterior cervical foraminotomy (PCF), posterior cervical laminectomy and anterior discectomy. Endoscopic PCF foraminotomy can be used to address a lateral disc herniation and osseous foraminal stenosis with far less muscle dissection and bony resection than open techniques (83,84). When comparing traditional anterior cervical decompression and fusion (ACDF) to open PCF for the treatment of lateral disc herniations, the literature has shown that both techniques have similar outcomes, complication profiles and index-level reoperation rates (85-90). Despite these similarities there has been an increasing number of cervical fusion procedures in the Unites States, which has been postulated to be due to procedural reimbursement (91). PCF has not only been shown to be cost-effective over ACDF (92,93), but also avoids fusion and fusion-related complications; the reoperation rate for adjacent segment disease is substantially more common after a fusion procedure, occurring in approximately 12% of patients undergoing ACDF (94) and only 2% to 3% after cervical foraminotomy (89). However, in exchange for avoiding fusion and ventral-approach related complications, open PCF entails stripping of the posterior cervical musculature with painful post-operative recovery, potential wound complications and risk of post-operative kyphosis (87,95). Minimally invasive techniques provide for comparable results with less blood loss, less pain requirements, faster recovery and shorter hospital stay over the standard open technique (96-98). Ruetten et al. conducted a prospective randomized controlled trial of 175 patients who underwent endoscopic PCF or ACDF with 2-year follow-up (99). The authors reported equivalent functional outcomes in both groups with no significant differences in the reoperation rate or number of overall complications. Notably, post-operative pain and post-operative work disability was significantly less in the endoscopic PCF group and the 3% complication rate in this cohort was limited to transient hypesthesia; furthermore, there was no radiographic evidence of increasing kyphosis or instability. Due to the maneuverability of the endoscope and the ability to manipulate optical field of view, we believe endoscopic PCF allows for a more thorough decompression of the cervical nerve root beyond the pedicle with minimal bony resection (Figure 7).
Similarly, minimally-invasive techniques have been shown to be valuable in the treatment of cervical myelopathy with the goal of performing a decompression procedure while minimizing the risk of post-laminectomy kyphosis (100). Minamide et al. recently compared five year clinical and radiologic outcomes following 78 patients who underwent either cervical endoscopically-assisted tubular laminotomy or conventional expansive laminoplasty and found similar JOA recovery rates and complication rates with significantly less blood loss, post-inflammatory markers and post-operative neck pain in the microendoscopic group (101). The most notable findings from this study is that the microendoscopic group not only had a lower incidence of post-operative kyphosis but there was a statistically significant greater gain in lordosis (+2.6°) when compared to the laminoplasty group (−1.2°) (P=0.031). Although there has been only one small case series reporting on outcomes following full-endoscopic decompression of patients with cervical myelopathy, the author reported favorable outcomes with no perioperative complications (102). Although other endoscopic cervical techniques include anterior cervical discectomy for disc herniation (103,104) and microendoscopic laminoplasty (105) have been reported, there is currently limited data regarding long-term outcomes and complication profiles. For moderate to higher complexity procedures, endoscopic spine surgery may offer significant benefits over traditional procedures, particularly if comparable clinical outcomes can be achieved while avoiding the morbidity associated with fusion and traditional open techniques.
Revision surgery and previous arthrodesis
One of the most significant advantages of endoscopic spine surgery is the ability to provide minimally invasive access to pathology in revision cases that would otherwise require extensive soft tissue dissection and significant scar mobilization. In revision lumbar cases with unilateral symptomology, utilizing the transforaminal approach can avoid epidural scar tissue from a previous interlaminar based decompression (106), hence, the morbidity associated with traditional revision microdiscectomy (107,108) can be circumvented. Ruetten et al. conducted a prospective, randomized, controlled study of 87 patients with recurrent lumbar disc herniations who underwent either full-endoscopic decompression (transforaminal or interlaminar) or conventional open revision discectomy with 2-year follow-up (109). While there were no significant differences in clinical outcomes and reoperation rates, the endoscopic group had significantly less overall complications (6% vs. 21%), less back pain, less post-operative pain requirements and less post-operative work disability. Endoscopic decompression techniques have also been shown to provide excellent clinical outcomes with low complication rates in patients with recurrent stenosis in the setting of previous arthrodesis (110,111). In the context of arthrodesis, endoscopic transforaminal interbody fusion has also been shown to be a feasible alternative to open and minimally invasive techniques, with satisfactory fusion rates and minimal morbidity (112). As endoscopic spine surgery continues to enjoy wider adoption, spine surgeons are expanding potential indications for use of this technology. Numerous case series have shown potential applications in patients with complex pathology including burst fractures (113), BMP related heterotopic ossification (114,115), synovial cysts (116,117), migrated hardware (115), discitis (118), spinal cord untethering (119), and tumors (120) (Figure 8). While direct comparisons to more traditional techniques for these complex scenarios is limited, current available evidence highlights the versatility of endoscopic spine surgery in achieving favorable outcome with minimal morbidity.
Conclusions
Endoscopic spine surgery has seen over 30 years of evolution and much has been learned from early iterations of these procedures. As per the Gartner technology adoption “hype cycle” model (121), there are patterns to social reactions and expectations in the context of the maturity, adoption and application of new technologies. When a new technology is first introduced, there are very high expectations with only minimal utilization. During this early “hype” period, newer technologies often are unable to meet overinflated expectations and are perceived to not provide improvement over the current standard. Larger adoption of technological innovation is only seen after a technology enters the “slope of enlightenment”, which represents a period in which the specific value of innovation is better understood. The “plateau of productivity” represents the final stage the technology life-cycle, whereby the true advantages and limitations of a new technology are accepted and a larger percentage of the population adopt a particular innovation. We believe that full-endoscopic spine surgery is currently in the “slope of enlightenment” phase of the technology adoption cycle as we are beginning to understand its role in spine surgery. In the context of an invasiveness and complexity index, we can better conceptualize the role of endoscopic spine surgery and understand its true utility in the treatment of spinal pathology to allow for more widespread adoption. Although there is a learning curve associated with these procedures, we believe that endoscopic techniques offer a more powerful and less morbid approach to spinal pathology that ultimately elevates the standard of care when treating our patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: CP Hofstetter: consulting and teaching for J&J, Globus and Joimax; R Hârtl: consultant brainlab DPS. S Hasan has no conflicts of interest to declare.
References
- Kambin P, Brager MD. Percutaneous posterolateral discectomy. Anatomy and mechanism. Clin Orthop Relat Res 1987.145-54. [PubMed]
- Gibson JNA, Subramanian AS, Scott CEH. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur Spine J 2017;26:847-56. [Crossref] [PubMed]
- Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976) 2006;31:E890-7. [Crossref] [PubMed]
- Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician 2015;18:61-70. [PubMed]
- Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine 2009;10:476-85. [Crossref] [PubMed]
- Tacconi L, Baldo S, Merci G, et al. Transforaminal percutaneous endoscopic lumbar discectomy: outcome and complications in 270 cases. J Neurosurg Sci 2018. [Epub ahead of print]. [PubMed]
- Zhang B, Liu S, Liu J, et al. Transforaminal endoscopic discectomy versus conventional microdiscectomy for lumbar discherniation: a systematic review and meta-analysis. J Orthop Surg Res 2018;13:169. [Crossref] [PubMed]
- Choi KC, Shim HK, Hwang JS, et al. Comparison of Surgical Invasiveness Between Microdiscectomy and 3 Different Endoscopic Discectomy Techniques for Lumbar Disc Herniation. World Neurosurg 2018;116:e750-8. [Crossref] [PubMed]
- Pan L, Zhang P, Yin Q. Comparison of tissue damages caused by endoscopic lumbar discectomy and traditional lumbar discectomy: a randomised controlled trial. Int J Surg 2014;12:534-7. [Crossref] [PubMed]
- Akçakaya MO, Yorukoglu AG, Aydoseli A, et al. Serum creatine phosphokinase levels as an indicator of muscle injury following lumbar disc surgery: Comparison of fully endoscopic discectomy and microdiscectomy. Clin Neurol Neurosurg 2016;145:74-8. [Crossref] [PubMed]
- Tao XZ, Jing L, Li JH. Therapeutic effect of transforaminal endoscopic spine system in the treatment of prolapse of lumbar intervertebral disc. Eur Rev Med Pharmacol Sci 2018;22:103-10. [PubMed]
- Chang F, Zhang T, Gao G, et al. Therapeutic effect of percutaneous endoscopic lumbar discectomy on lumbar disc herniation and its effect on oxidative stress in patients with lumbar disc herniation. Exp Ther Med 2018;15:295-9. [PubMed]
- Parihar VS, Yadav N, Ratre S, et al. Endoscopic Anterior Approach for Cervical Disc Disease (Disc Preserving Surgery). World Neurosurg 2018;115:e599-609. [Crossref] [PubMed]
- Wu PF, Liu BH, Wang B, et al. Complications of Full-Endoscopic Versus Microendoscopic Foraminotomy for Cervical Radiculopathy: A Systematic Review and Meta-Analysis. World Neurosurg 2018;114:217-27. [Crossref] [PubMed]
- Ruetten S, Hahn P, Oezdemir S, et al. Operation of Soft or Calcified Thoracic Disc Herniations in the Full-Endoscopic Uniportal Extraforaminal Technique. Pain Physician 2018;21:E331-40. [PubMed]
- Ruetten S, Hahn P, Oezdemir S, et al. Full-endoscopic uniportal decompression in disc herniations and stenosis of the thoracic spine using the interlaminar, extraforaminal, or transthoracic retropleural approach. J Neurosurg Spine 2018;29:157-68. [Crossref] [PubMed]
- Scott WW Jr. The development of the cystoscope. From "lichtleiter" to fiber optics. Invest Urol 1969;6:657-61. [PubMed]
- Jackson RW. A history of arthroscopy. Arthroscopy 2010;26:91-103. [Crossref] [PubMed]
- Snyder LA, O'Toole J, Eichholz KM, et al. The technological development of minimally invasive spine surgery. Biomed Res Int 2014;2014:293582. [Crossref] [PubMed]
- Craig FS. The Craig vertebral body biopsy. N Y State J Med 1955;55:3422-4. [PubMed]
- Ottolenghi CE. Diagnosis of orthopaedic lesions by aspiration biopsy; results of 1,061 punctures. J Bone Joint Surg Am 1955;37-A:443-64. [Crossref] [PubMed]
- Valls J, Ottolenghi CE, Schajowicz F. Aspiration biopsy in diagnosis of lesions of vertebral bodies. J Am Med Assoc 1948;136:376-82. [Crossref] [PubMed]
- Kambin P. Arthroscopic Microdiscectomy: Minimal Intervention Spinal Surgery. Baltimore, MD: Urban & Schwarzenberg, 1990.
- Hijikata S, Yamagishi M, Nakayma T. Percutaneous discectomy: a new treatment method for lumbar disc herniation. J Toden Hosp 1975;5:5-13.
- Kambin P, Schaffer JL. Percutaneous lumbar discectomy. Review of 100 patients and current practice. Clin Orthop Relat Res 1989.24-34. [Crossref] [PubMed]
- Kambin P, Zhou L. History and current status of percutaneous arthroscopic disc surgery. Spine (Phila Pa 1976) 1996;21:57S-61S. [Crossref] [PubMed]
- Onik G, Helms CA, Ginsberg L, et al. Percutaneous lumbar diskectomy using a new aspiration probe: porcine and cadaver model. Radiology 1985;155:251-2. [Crossref] [PubMed]
- Schaffer JL, Kambin P. Percutaneous posterolateral lumbar discectomy and decompression with a 6.9-millimeter cannula. Analysis of operative failures and complications. J Bone Joint Surg Am 1991;73:822-31. [Crossref] [PubMed]
- Kambin P. Diagnostic and therapeutic spinal arthroscopy. Neurosurg Clin N Am 1996;7:65-76. [Crossref] [PubMed]
- Mathews HH. Transforaminal endoscopic microdiscectomy. Neurosurg Clin N Am 1996;7:59-63. [Crossref] [PubMed]
- Yeung AT. The Evolution and Advancement of Endoscopic Foraminal Surgery: One Surgeon's Experience Incorporating Adjunctive Techologies. SAS J 2007;1:108-17. [Crossref] [PubMed]
- Kambin P. Arthroscopic microdiscectomy of the lumbar spine. Clin Sports Med 1993;12:143-50. [PubMed]
- Schreiber A, Suezawa Y, Leu H. Does percutaneous nucleotomy with discoscopy replace conventional discectomy? Eight years of experience and results in treatment of herniated lumbar disc. Clin Orthop Relat Res 1989.35-42. [Crossref] [PubMed]
- Suezawa Y, Jacob HA, Schreiber A. Percutaneous nucleotomy. An alternative to spinal surgery for lumbar disc herniation. Acta Orthop Belg 1987;53:293-9. [PubMed]
- Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol 2005;17:641-61. [Crossref] [PubMed]
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999;21:39-42. [Crossref] [PubMed]
- Smith MM, Foley KT. Microendoscopic Discectomy (MED): The First 100 Cases. Neurosurgery 1998;43:702. [Crossref]
- Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res 1986.37-43. [PubMed]
- Ruetten S, Komp M, Merk H, et al. Use of newly developed instruments and endoscopes: full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine 2007;6:521-30. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931-9. [Crossref] [PubMed]
- Kim M, Lee S, Kim HS, et al. A Comparison of Percutaneous Endoscopic Lumbar Discectomy and Open Lumbar Microdiscectomy for Lumbar Disc Herniation in the Korean: A Meta-Analysis. Biomed Res Int 2018;2018:9073460. [Crossref] [PubMed]
- Qin R, Liu B, Hao J, et al. Percutaneous Endoscopic Lumbar Discectomy Versus Posterior Open Lumbar Microdiscectomy for the Treatment of Symptomatic Lumbar Disc Herniation: A Systemic Review and Meta-Analysis. World Neurosurg 2018;120:352-62. [Crossref] [PubMed]
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA 2006;296:2441-50. [Crossref] [PubMed]
- Kim CH, Chung CK, Park CS, et al. Reoperation rate after surgery for lumbar herniated intervertebral disc disease: nationwide cohort study. Spine (Phila Pa 1976) 2013;38:581-90. [Crossref] [PubMed]
- Carragee EJ, Han MY, Suen PW, et al. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am 2003;85:102-8. [Crossref] [PubMed]
- Abdu RW, Abdu WA, Pearson AM, et al. Reoperation for Recurrent Intervertebral Disc Herniation in the Spine Patient Outcomes Research Trial: Analysis of Rate, Risk Factors, and Outcome. Spine (Phila Pa 1976) 2017;42:1106-14. [Crossref] [PubMed]
- Martens F, Vajkoczy P, Jadik S, et al. Patients at the Highest Risk for Reherniation Following Lumbar Discectomy in a Multicenter Randomized Controlled Trial. JB JS Open Access 2018;3:e0037.
- McGirt MJ, Ambrossi GL, Datoo G, et al. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery 2009;64:338-44; discussion 344-5. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Feltes CH, et al. Correlation of the amount of disc removed in a lumbar microdiscectomy with long-term outcome. Spine (Phila Pa 1976) 2004;29:2521-4; discussion 2525-6. [Crossref] [PubMed]
- Chen Z, Zhang L, Dong J, et al. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1-year results of an ongoing randomized controlled trial. J Neurosurg Spine 2018;28:300-10. [Crossref] [PubMed]
- Sen RD, White-Dzuro G, Ruzevick J, et al. Intra- and Perioperative Complications Associated with Endoscopic Spine Surgery: A Multi-Institutional Study. World Neurosurg 2018;120:e1054-60. [Crossref] [PubMed]
- Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976) 2007;32:1-8. [Crossref] [PubMed]
- Atlas SJ, Keller RB, Robson D, et al. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine study. Spine (Phila Pa 1976) 2000;25:556-62. [Crossref] [PubMed]
- Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976) 2005;30:936-43. [Crossref] [PubMed]
- Parker SL, Fulchiero EC, Davis BJ, et al. Cost-effectiveness of multilevel hemilaminectomy for lumbar stenosis-associated radiculopathy. Spine J 2011;11:705-11. [Crossref] [PubMed]
- Parker SL, Godil SS, Mendenhall SK, et al. Two-year comprehensive medical management of degenerative lumbar spine disease (lumbar spondylolisthesis, stenosis, or disc herniation): a value analysis of cost, pain, disability, and quality of life: clinical article. J Neurosurg Spine 2014;21:143-9. [Crossref] [PubMed]
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008.794-810. [Crossref] [PubMed]
- Wilby MJ, Seeley H, Laing RJ. Laminectomy for lumbar canal stenosis: a safe and effective treatment. Br J Neurosurg 2006;20:391-5. [Crossref] [PubMed]
- Jönsson B, Annertz M, Sjoberg C, et al. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part II: Five-year follow-up by an independent observer. Spine (Phila Pa 1976) 1997;22:2938-44. [Crossref] [PubMed]
- Fox MW, Onofrio BM, Onofrio BM, et al. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg 1996;85:793-802. [Crossref] [PubMed]
- Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 1991;73:802-8. [Crossref] [PubMed]
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 2010;35:1329-38. [Crossref] [PubMed]
- Guha D, Heary RF, Shamji MF. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg Focus 2015;39:E9. [Crossref] [PubMed]
- Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N Engl J Med 2016;374:1424-34. [Crossref] [PubMed]
- Försth P, Olafsson G, Carlsson T, et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med 2016;374:1413-23. [Crossref] [PubMed]
- Hamasaki T, Tanaka N, Kim J, et al. Biomechanical assessment of minimally invasive decompression for lumbar spinal canal stenosis: a cadaver study. J Spinal Disord Tech 2009;22:486-91. [Crossref] [PubMed]
- Hasegawa K, Kitahara K, Shimoda H, et al. Biomechanical evaluation of destabilization following minimally invasive decompression for lumbar spinal canal stenosis. J Neurosurg Spine 2013;18:504-10. [Crossref] [PubMed]
- Mobbs RJ, Li J, Sivabalan P, et al. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine 2014;21:179-86. [Crossref] [PubMed]
- Kato M, Namikawa T, Matsumura A, et al. Radiographic Risk Factors of Reoperation Following Minimally Invasive Decompression for Lumbar Canal Stenosis Associated With Degenerative Scoliosis and Spondylolisthesis. Global Spine J 2017;7:498-505. [Crossref] [PubMed]
- Kelleher MO, Timlin M, Persaud O, et al. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine (Phila Pa 1976) 2010;35:E981-7. [Crossref] [PubMed]
- Daubs MD, Lenke LG, Bridwell KH, et al. Decompression alone versus decompression with limited fusion for treatment of degenerative lumbar scoliosis in the elderly patient. Evid Based Spine Care J 2012;3:27-32. [PubMed]
- Transfeldt EE, Topp R, Mehbod AA, et al. Surgical outcomes of decompression, decompression with limited fusion, and decompression with full curve fusion for degenerative scoliosis with radiculopathy. Spine (Phila Pa 1976) 2010;35:1872-5. [Crossref] [PubMed]
- Simpson JM, Silveri CP, Simeone FA, et al. Thoracic disc herniation. Re-evaluation of the posterior approach using a modified costotransversectomy. Spine (Phila Pa 1976) 1993;18:1872-7. [Crossref] [PubMed]
- Patterson RH Jr, Arbit E. A surgical approach through the pedicle to protruded thoracic discs. J Neurosurg 1978;48:768-72. [Crossref] [PubMed]
- Larson SJ, Holst RA, Hemmy DC, et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg 1976;45:628-37. [Crossref] [PubMed]
- Ransohoff J, Spencer F, Siew F, et al. Transthoracic removal of thoracic disc. Report of three cases. J Neurosurg 1969;31:459-61. [Crossref] [PubMed]
- Anand N, Regan JJ. Video-assisted thoracoscopic surgery for thoracic disc disease: Classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine (Phila Pa 1976) 2002;27:871-9. [Crossref] [PubMed]
- Fessler RG, Sturgill M. Review: complications of surgery for thoracic disc disease. Surg Neurol 1998;49:609-18. [Crossref] [PubMed]
- Faciszewski T, Winter RB, Lonstein JE, et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1223 procedures. Spine (Phila Pa 1976) 1995;20:1592-9. [Crossref] [PubMed]
- Sundaresan N, Shah J, Foley KM, et al. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg 1984;61:686-90. [Crossref] [PubMed]
- Choi KY, Eun SS, Lee SH, et al. Percutaneous endoscopic thoracic discectomy; transforaminal approach. Minim Invasive Neurosurg 2010;53:25-8. [Crossref] [PubMed]
- Nie HF, Liu KX. Endoscopic transforaminal thoracic foraminotomy and discectomy for the treatment of thoracic disc herniation. Minim Invasive Surg 2013;2013:264105. [Crossref] [PubMed]
- Nakamura S, Taguchi M. Area of Ostectomy in Posterior Percutaneous Endoscopic Cervical Foraminotomy: Images and Mid-term Outcomes. Asian Spine J 2017;11:968-74. [Crossref] [PubMed]
- Ye ZY, Kong WJ, Xin ZJ, et al. Clinical Observation of Posterior Percutaneous Full-Endoscopic Cervical Foraminotomy as a Treatment for Osseous Foraminal Stenosis. World Neurosurg 2017;106:945-52. [Crossref] [PubMed]
- Herkowitz HN, Kurz LT, Overholt DP. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine (Phila Pa 1976) 1990;15:1026-30. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Jagannathan J, Sherman JH, Szabo T, et al. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years' clinical and radiographic follow-up. J Neurosurg Spine 2009;10:347-56. [Crossref] [PubMed]
- Wirth FP, Dowd GC, Sanders HF, et al. Cervical discectomy. A prospective analysis of three operative techniques. Surg Neurol 2000;53:340-6; discussion 346-8. [Crossref] [PubMed]
- Clarke MJ, Ecker RD, Krauss WE, et al. Same-segment and adjacent-segment disease following posterior cervical foraminotomy. J Neurosurg Spine 2007;6:5-9. [Crossref] [PubMed]
- Lubelski D, Healy AT, Silverstein MP, et al. Reoperation rates after anterior cervical discectomy and fusion versus posterior cervical foraminotomy: a propensity-matched analysis. Spine J 2015;15:1277-83. [Crossref] [PubMed]
- Wang MC, Kreuter W, Wolfla CE, et al. Trends and variations in cervical spine surgery in the United States: Medicare beneficiaries, 1992 to 2005. Spine (Phila Pa 1976) 2009;34:955-61; discussion 962-3. [Crossref] [PubMed]
- Mansfield HE, Canar WJ, Gerard CS, et al. Single-level anterior cervical discectomy and fusion versus minimally invasive posterior cervical foraminotomy for patients with cervical radiculopathy: a cost analysis. Neurosurg Focus 2014;37:E9. [Crossref] [PubMed]
- Tumialán LM, Ponton RP, Gluf WM. Management of unilateral cervical radiculopathy in the military: the cost effectiveness of posterior cervical foraminotomy compared with anterior cervical discectomy and fusion. Neurosurg Focus 2010;28:E17. [Crossref] [PubMed]
- Xu R, Bydon M, Macki M, et al. Adjacent segment disease after anterior cervical discectomy and fusion: clinical outcomes after first repeat surgery versus second repeat surgery. Spine (Phila Pa 1976) 2014;39:120-6. [Crossref] [PubMed]
- Cağlar YS, Bozkurt M, Kahilogullari G, et al. Keyhole approach for posterior cervical discectomy: experience on 84 patients. Minim Invasive Neurosurg 2007;50:7-11. [Crossref] [PubMed]
- Skovrlj B, Gologorsky Y, Haque R, et al. Complications, outcomes, and need for fusion after minimally invasive posterior cervical foraminotomy and microdiscectomy. Spine J 2014;14:2405-11. [Crossref] [PubMed]
- Clark JG, Abdullah KG, Steinmetz MP, et al. Minimally Invasive versus Open Cervical Foraminotomy: A Systematic Review. Global Spine J 2011;1:9-14. [Crossref] [PubMed]
- Winder MJ, Thomas KC. Minimally invasive versus open approach for cervical laminoforaminotomy. Can J Neurol Sci 2011;38:262-7. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:940-8. [Crossref] [PubMed]
- Lonstein JE. Post-laminectomy kyphosis. Clin Orthop Relat Res 1977.93-100. [PubMed]
- Minamide A, Yoshida M, Simpson AK, et al. Microendoscopic laminotomy versus conventional laminoplasty for cervical spondylotic myelopathy: 5-year follow-up study. J Neurosurg Spine 2017;27:403-9. [Crossref] [PubMed]
- Shen J. Fully Endoscopic Bilateral Cervical Laminotomy with Unilateral Approach for Cervical Spinal Stenosis and Myelopathy: A Case Series. J Spine 2018;S7:009.
- Yang JS, Chu L, Chen L, et al. Anterior or posterior approach of full-endoscopic cervical discectomy for cervical intervertebral disc herniation? A comparative cohort study. Spine (Phila Pa 1976) 2014;39:1743-50. [Crossref] [PubMed]
- Du Q, Wang X, Qin JP, et al. Percutaneous Full-Endoscopic Anterior Transcorporeal Procedure for Cervical Disc Herniation: A Novel Procedure and Early Follow-Up Study. World Neurosurg 2018;112:e23-30. [Crossref] [PubMed]
- Zhang C, Li D, Wang C, et al. Cervical Endoscopic Laminoplasty for Cervical Myelopathy. Spine (Phila Pa 1976) 2016;41 Suppl 19:B44-51. [Crossref] [PubMed]
- Lewandrowski KU. Endoscopic Transforaminal and Lateral Recess Decompression After Previous Spinal Surgery. Int J Spine Surg 2018;12:98-111. [Crossref] [PubMed]
- Suk KS, Lee HM, Moon SH, et al. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976) 2001;26:672-6. [Crossref] [PubMed]
- Ozgen S, Naderi S, Ozek MM, et al. Findings and outcome of revision lumbar disc surgery. J Spinal Disord 1999;12:287-92. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Recurrent lumbar disc herniation after conventional discectomy: a prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech 2009;22:122-9. [Crossref] [PubMed]
- Wu JJ, Chen HZ, Zheng C. Transforaminal Percutaneous Endoscopic Discectomy and Foraminoplasty after Lumbar Spinal Fusion Surgery. Pain Physician 2017;20:E647-51. [PubMed]
- McGrath LB Jr, Madhavan K, Chieng LO, et al. Early experience with endoscopic revision of lumbar spinal fusions. Neurosurg Focus 2016;40:E10. [Crossref] [PubMed]
- Wang MY, Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg Focus 2016;40:E13. [Crossref] [PubMed]
- Wang Y, Ning C, Yao L, et al. Transforaminal endoscopy in lumbar burst fracture: A case report. Medicine (Baltimore) 2017;96:e8640. [Crossref] [PubMed]
- Telfeian AE. An awake, minimally-invasive, fully-endoscopic surgical technique for treating lumbar radiculopathy secondary to heterotopic foraminal bone formation after a minimally invasive transforaminal lumbar interbody fusion with BMP: technical note. J Spine Surg 2018;4:162-6. [Crossref] [PubMed]
- Wagner R, Iprenburg M, Telfeian AE. Transforaminal endoscopic decompression of a postoperative dislocated bone fragment after a 2-level lumbar total disc replacement: case report. Neurosurg Focus 2016;40:E8. [Crossref] [PubMed]
- Wu HH, Chu L, Zhu Y, et al. Percutaneous Endoscopic Lumbar Surgery via the Transfacet Approach for Lumbar Synovial Cyst. World Neurosurg 2018;116:35-9. [Crossref] [PubMed]
- Oertel JM, Burkhardt BW. Endoscopic Surgical Treatment of Lumbar Synovial Cyst: Detailed Account of Surgical Technique and Report of 11 Consecutive Patients. World Neurosurg 2017;103:122-32. [Crossref] [PubMed]
- Ito M, Abumi K, Kotani Y, et al. Clinical outcome of posterolateral endoscopic surgery for pyogenic spondylodiscitis: results of 15 patients with serious comorbid conditions. Spine (Phila Pa 1976) 2007;32:200-6. [Crossref] [PubMed]
- Telfeian AE, Punsoni M, Hofstetter CP. Minimally invasive endoscopic spinal cord untethering: case report. J Spine Surg 2017;3:278-82. [Crossref] [PubMed]
- Telfeian AE, Choi DB, Aghion DM. Transforaminal endoscopic surgery under local analgesia for ventral epidural thoracic spinal tumor: Case report. Clin Neurol Neurosurg 2015;134:1-3. [Crossref] [PubMed]
- Fenn J RM. Mastering the hype cycle. Brighton, MA: Harvard Business Press; 2008.


