
Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF
Introduction
Lumbar interbody fusion (LIF) is an established treatment for a range of spinal disorders including; degenerative pathologies, trauma, infection and neoplasia (1). LIF involves placement of an implant (cage, spacer or structural graft) within the intervertebral space after discectomy and endplate preparation. At this time LIF is performed using five main approaches; posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF or MI-TLIF), oblique lumbar interbody fusion/anterior to psoas (OLIF/ATP), anterior lumbar interbody fusion (ALIF) and lateral lumbar interbody fusion (LLIF) (Figure 1A,B). There is no clear definitive evidence for one approach being superior to another in terms of fusion or clinical outcomes. These operations can also be performed using mini-open or minimally invasive (MIS) approaches (2,3). Interbody fusion is preferable to postero-lateral ‘on-lay’ fusion techniques due to lower rates of postoperative complications and pseudoarthrosis (2).
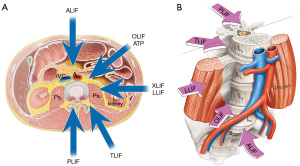
Patient expectations and increasing demands for shorter hospital stay and early return to work has led to more innovative surgical techniques to reduce iatrogenic injury and postoperative morbidity. The growth of new techniques attempts to shorten operative times and achieve faster recovery with reduced operative complications (4). Initial descriptions of ALIF and PLIF have been challenged by the evolving alternate approaches, such as the transforaminal, lateral and more recently oblique techniques. We discuss the various zones of approach by defining anterior options as any approach that is anterior to the Transverse Process and involves a retroperitoneal corridor to the lumbar spine (ALIF/OLIF/LLIF), and posterior options being an approach posterior to the Transverse Process, involving traversing the spinal canal or foramen for access to the disc and interbody space (PLIF/TLIF/MI-TLIF).
Since the initial description of the PLIF technique by Briggs and Milligan in 1944 (5), the method of PLIF has evolved, with the development of additional options of autologous and synthetic bone grafting, advanced methods of spinal segmental fusion techniques, innovative implants including the wide variety of interbody implants we use today and the use of pedicle screw fixation for posterior instrumentation. With advances in implants and techniques, the results of spinal fusion for PLIF has improved. Harms and Rolinger reported a newer technique in 1982 via the transforaminal route to achieve the insertion of an interbody cage packed with bone graft, termed TLIF (6). This created another option for surgeons in their armamentarium for treatment of patients with symptomatic disc disease, spondylolisthesis and degenerative lumbar scoliosis.
Technique review
PLIF
One of the original approaches for LIF is PLIF. In the PLIF technique, surgical access to the intervertebral disc is gained from a posterior direction. The patient is initially positioned in a prone position on an Andrews or Jackson table. Either an open midline approach with bilateral muscle strip dissection or MIS paramedian Wiltse muscle splitting approach can be used to access the posterior column of the vertebral body. Once the spinous process and laminae at the appropriate levels are identified (L1-S1), a laminotomy may be performed medial to the facet and the dura retracted to exposure a corridor to the disc space. The endplates and disc space can then be prepared to allow implant/spacer insertion.
The posterior approach may be suitable for degenerative indications requiring a fusion procedure. Selected patients with segmental instability, recurrent disc herniation, symptomatic spinal stenosis and pseudoarthrosis may also benefit from a PLIF procedure. Contraindications for posterior fusion surgery include extensive epidural scarring, arachnoiditis, and active infection.
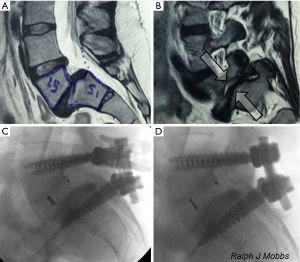
There are several advantages associated with PLIF surgery. Firstly, the PLIF approach is a traditional lumbar approach that the majority of spinal surgeons are well trained and comfortable in performing. A posterior exposure allows excellent visualization of the nerve roots without compromising blood supply to the graft. PLIF allows for adequate interbody height restoration, allows for neural decompression whilst maintaining posterior support structures (7). Furthermore, posterior fusion surgery also allows for potential 360-degree fusion through a single incision. There are disadvantages that a surgeon should be wary of when performing PLIF (8). Firstly, there may be significant paraspinal iatrogenic injury associated with prolonged muscle retraction (9). This may delay recovery and mobilization due to approach-related muscle trauma. Using this technique, it may be difficult to correct coronal imbalance and restore lordosis. Endplate preparation may be difficult compared to anterior fusion approaches. Other potential risks include retraction injury of nerve roots causing fibrosis and chronic radiculopathy (10-12) (Figure 2).
TLIF
Another posterior surgical approach for fusion is TLIF, used for stabilization and treatment of degenerative lumbar disease following failed conservative treatment. The main concerns with the posterior fusion approach was the extent of neural retraction required, with particular concerns surrounding potential nerve root injury, dural tears and epidural fibrosis. To address this limitation, the TLIF approach was proposed, involving direct, unilateral access to the intervertebral foraminal space whilst reducing direct dissection and surgical trauma to spinal muscles and structural integrity. By opening the neural foramen on one side only, damage to important anatomical structures such as nerve roots, dura and ligamentum flavum may be reduced. Like other fusion procedures, TLIF can be performed via an open procedure or MIS “mini-open” technique with smaller incision sizes and use of microscopy.
The TLIF approach involves positioning the patient prone after the patient is put under general anesthesia. A midline or bilateral paramedian mini-open incision is used, allowing access to the disc space suitable for levels L1-S1. The spinal canal is entered via a unilateral laminectomy and inferior facetectomy, which facilitates bone graft placement.
Indications of a TLIF approach include all degenerative pathologies, including broad-based disc prolapses, degenerate disc disease, recurrent disc herniation, pseudoarthrosis, and symptomatic spondylosis. Contraindications are similar to PLIF and include extensive epidural scaring, arachnoiditis, active infection and conjoined nerve roots (that may preclude access to the disc space) and osteoporotic patients.
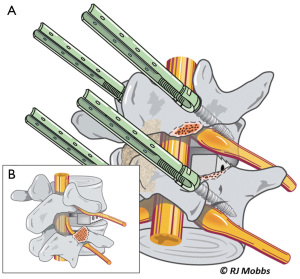
Advantages of the TLIF approach include relatively easier access to the posterior structures including the lamina, ligamentum flavum and facet joints. Compared to a traditional PLIF technique, the TLIF approach preserves ligamentous structures which are instrumental to restoring biomechanical stability of the segment and adjacent structures (10,13-15). In TLIF, a single unilateral incision is able to provide bilateral anterior column support. Access using a MIS mini-open incision and magnification (loupes or microscope) may further reduce access-associated muscle injury, minimize bleeding and improve postoperative recovery. The disadvantage is that TLIF, like PLIF, is associated with significant paraspinal iatrogenic injury with prolonged muscle retraction. It may be difficult to correct coronal imbalance and restore lordosis (11,16,17). Compared to anterior approaches, endplate preparation may be difficult (Figure 3).
ALIF
Particularly in the realm of discogenic low back pain, ALIF has evolved to be an efficacious and predominant surgical technique. The anterior retroperitoneal approach facilitates adequate access to the entire ventral surface of the exposed disc, allowing comprehensive discectomy and direct implant insertion. For this technique, the patient is prepared and positioned supine. Incision and approach include midline, paramedian (all levels) or Mini-Pfannenstiel (L5/S1) incision with a retroperitoneal corridor and vascular mobilization and dissection. The ALIF approach is suitable for levels L4/L5 and L5/S1, primarily the latter due to vascular anatomy (Figure 4).The ALIF approach is limited for L2/3 and L3/4 secondary to extensive peritoneal and kidney (L2/3) retraction and the risk of superior mesenteric artery thrombosis, although rare.
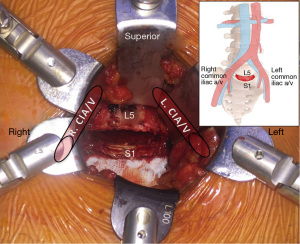
An ALIF procedure may be suitable for degenerative disc disease, discogenic disease and revision of failed posterior fusion (18). Contraindications of ALIF include significant prior abdominal surgery with adhesions or adverse vascular anatomy, severe peripheral vascular disease, solitary kidney on the side of exposure, spinal infection and high-grade (Grade 2+) degenerative spondylolisthesis in the absence of posterior fusion (19). Isthmic spondylolisthesis at L5/S1 is a relative contraindication (20,21) and should include posterior fixation in combination with the ALIF technique.
The ALIF approach is associated with several key advantages. Firstly, this technique allows direct midline view of the disc space and extensive lateral exposure of the vertebral bodies, which permits efficient disc space clearance with rapid endplate preparation. Furthermore, the anterior access allows maximization of the implant size and surface area, which facilitates aggressive correction of lordosis and foraminal height restoration. This may lead to high fusion rates with ample disc space preparation (13,14,19,22,23). ALIF also allows sparing of posterior spinal muscles and anterolateral psoas muscles, which may reduce postoperative pain and disability. Disadvantages of the ALIF technique include approach-related complications such as retrograde ejaculation, visceral and vascular injury (14,19,24).
LLIF
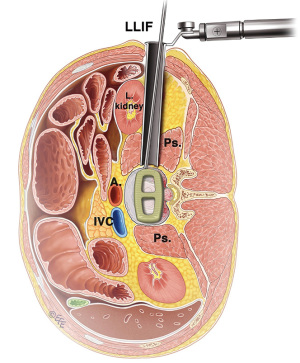
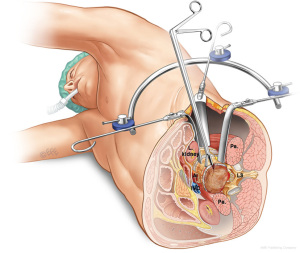
The LLIF or extreme lateral interbody fusion (XLIF) technique was described by Ozgur et al. in 2006 (25) involves accessing the disc space via a lateral retroperitoneal, transpsoas corridor. LLIF is suitable for conditions that require access to the interbody disc space from T12/L1 to L4/5. This technique is not suitable for the L5/S1 level, due to the location of the iliac crest that obstructs lateral access. Furthermore, at more caudal levels of the lumbar spine, the lumbar plexus courses more anteriorly and the iliac vessels course more laterally, which increases risk of injury via a lateral approach. The patient is positioned laterally, either left or right side up depending on surgeon’s preference and ease of access. A small lateral incision is performed based on position and angulation of the disc on image intensification when the patient is positioned. Neuromonitoring is essential for the transpsoas access to the disc space.
The LLIF approach is suitable for all degenerative indications. It is an excellent option for sagittal and coronal deformity correction, especially for lumbar degenerative scoliosis with laterolisthesis (26). However, the LLIF approach may not be suitable for severe central canal stenosis, bony lateral recess stenosis and high-grade spondylolisthesis (27). For operators using a standalone LLIF approach without posterior instrumentation, this should not be used under high biomechanical stress such as facet arthropathy, instability, deformity, adjacent to a previous fusion and multiple levels (28). The lateral approach is also not suitable in patients with prior retroperitoneal surgery or with retroperitoneal abscess, as well as patients with abnormal vascular anatomy. Advantages of LLIF are a MIS muscle-splitting approach that can be performed with rapid postoperative mobilization. Aggressive deformity correction can be achieved with high fusion rates and comprehensive disc space clearance (2,4). Disadvantages include potential risks of lumbar plexus, psoas muscle and bowel injury, particularly at the L4/5 level (29). Vascular injury, if it occurs, may be difficult to control and represents another risk of the lateral transpsoas approach (26,30,31) (Figure 5).
OLIF/ATP
The OLIF or ATP approach was first described by Michael Mayer in 1977 (32) and involves an MIS access to the disc space via a corridor between the peritoneum and psoas muscle (Figure 6). Similarly to an LLIF approach, OLIF does not require posterior surgery, laminectomy, facetectomy or stripping of spinal or paraspinal musculature. However in contrast to the lateral transpsoas approach, the OLIF technique does not dissect or traverse the psoas muscle. For this technique, the patient is positioned laterally, either left or right side up depending on the surgeon’s preference and ease of access (33,34). A lateral and paramedian incision is performed based on position and angulation of the disc on image intensification when the patient is positioned (35). Neuromonitoring is not necessary as the anatomical corridor anterior to the psoas muscle is used for access. The OLIF technique is suitable for levels L1-S1.
Indications for OLIF include all degenerative indications. Similar to LLIF, OLIF is excellent for sagittal and coronal deformity correction, especially lumbar degenerative scoliosis with latero-listhesis. The OLIF approach is contraindicated in patients with severe central canal stenosis and high grade spondylolisthesis.
Advantages of the OLIF approach include that it facilitates MIS surgery with rapid postoperative mobilization. OLIF also allows aggressive deformity correction, high fusion rates with comprehensive disc space clearance (33,34,36). Lumbar plexus and psoas injury are unlikely as dissection is performed anterior to the psoas. However, potential risks involved with OLIF surgery include sympathetic dysfunction and vascular injury (36).
Part 1. Comparative evidence based on interbody fusion technique
ALIF vs. TLIF
Carefully selected patients with degenerative disc disease and spinal deformities are amenable to surgical intervention with ALIF or TLIF. While both approaches involve removal of the degenerative disc and insertion of an interbody device, the anterior and posterior approaches are both associated with their own unique benefits and challenges.
The current evidence comparing ALIF and TLIF remains mostly limited to observational retrospective evidence. To the best of our knowledge, there has been no published multi-center prospective randomized controlled trial comparing anterior and posterior fusion surgery, for either single or multilevel pathologies. One of the earliest comparative studies was reported by Hacker et al. (37), who compared 21 ALIF patients versus 54 TLIF patients who had ‘discogenic’ low back pain. Successful fusion rate was significantly higher in the TLIF group (53 out of 54 patients) compared to ALIF (16 out of 21 patients). One criticism of this study, however, was that it represents an early experience of an approach used by few groups, with higher allograft malposition rates (3 out of 53 patients) which may be attributed to a learning curve of this technique. More recent studies by Faundez et al. (38), Kim et al. (39) and Dorward et al. (40) did not show any significant difference in fusion rates between ALIF versus TLIF approaches.
In the context of a lack of randomized evidence, the next best level of evidence is a systematic review and meta-analysis of retrospective comparative evidence. In a recent meta-analysis (14), no significant difference in fusion rates was demonstrated between ALIF versus TLIF (88.6% vs. 91.9%, P=0.23). Dural injury was found to be significantly lower in the ALIF group (0.4% vs. 3.8%), blood vessel injury significantly higher in the ALIF group (2.6% vs. 0%), whilst no differences were detected in terms of neurological deficit (6.8% vs. 7.9%) and infection rates (4.9% vs. 4.3%).
Few of the comparative studies reported radiographic outcomes. Hsieh et al. reported significantly higher disc height, segmental lordosis and total lumbar lordosis in the ALIF group compared with TLIF (13). These trends were corroborated by Kim et al. in 2009 and 2010. In 2015, a standardized foramen measurement technique called the “pedicle-to-pedicle” technique was used to show that ALIF was associated with significant indirect foraminal decompression, with restoration of disc height and foraminal height (23).
PLIF vs. TLIF
PLIF and TLIF are both commonly used posterior approaches for fusion surgery, and represent the majority of cases preformed worldwide in terms of LIF (41-44). First described in 1940 by Cloward, PLIF allows three-column fixation with 360° fusion and anterior support (45,46). The TLIF procedure introduced in 1982, involves placement of pedicle screws and an interbody spacer via a posterolateral route through a facetectomy corridor (6). The advantages, disadvantages and indications for these techniques are described above.
Multiple studies have attempted to compare PLIF versus TLIF in terms of clinical and fusion outcomes. Humphreys et al. compared 34 PLIF vs. 40 TLIF patients in their report in 2001 (11). There were 10 complications in the PLIF group compared to 0 events in the TLIF group. Graft malposition occurred in four patients in the PLIF group, but none were reported in the TLIF group. Park et al. in 2005 reported 7 patients out of 99 PLIF patients with complications, compared to 0 events out of 29 TLIF patients (10). More recent studies such as Audat et al. (2012) and Sakeb et al. (2013) have also demonstrated similar trends (15,16). Radiographic fusion rates have been reported in fewer studies. Yan et al. (2008) and Zhuo et al. (2009) both reported 100% radiographic fusion rates for PLIF and TLIF (47,48). Audat et al. (15) and Sakeb et al. (16) reported no significant differences in radiographic fusion rates between PLIF and TLIF.
To summarize the available evidence, a systematic review and meta-analysis was conducted by Zhang et al. (12). From seven comparative studies, PLIF was associated with a higher complication (P<0.00001) whilst TLIF was associated with reduced durotomy (P=0.01). Clinical satisfaction (P=0.54), blood loss (P=0.14), nerve root injury (P=0.08), graft malposition (P=0.06), infection (P=0.36) and rate of radiographic fusion (P=0.27) were similar between PLIF versus TLIF cohorts. PLIF however required longer operative time (P=0.03). As such, the evidence suggests that TLIF could reduce the complication rate and durotomy rate whilst shortening duration of procedure. Neither TLIF nor PLIF was found superior in terms of clinical satisfaction or radiographic fusion rate (12).
Recent studies have also explored MIS options for PLIF (49) and TLIF (49). An open approach may be associated with improved deformity reduction with bilateral facetectomy possible. However, MIS PLIF/TLIF may be associated with reduced blood loss and shorter hospital stay, albeit longer operative times. Evidence to date has not demonstrated any differences in patient-related outcomes when comparing open versus MIS approaches, and requires further validation in prospective randomized controlled trials (50). MIS approaches may be more suitable for single level pathologies, again with robust data lacking to confirm this statement.
LLIF vs. ALIF/TLIF/PSF
Given the recent introduction of LLIF and more recently OLIF approaches, there have been few comparative studies reporting clinical and radiographic outcomes of these techniques to traditional approaches. In 2015, Sembrano et al. compared ALIF, LLIF, TLIF and PSF (posterior spinal fusion—no interbody device inserted) in terms of 6-week radiographic outcomes (51). The primary research question of this study of 147 patients (212 levels) was: how does LLIF compare to standard fusion approaches (ALIF, TLIF and PSF) in terms of improving segmental lordosis at the operative level(s). Secondary questions examined include how LLIF compared to standard approaches in terms of change in operative level(s) disc heights as well as regional lumbar lordosis. The operative numbers were relatively low including LLIF (35 patients; 54 levels); ALIF (36 patients; 57 levels); TLIF (50 patients; 65 levels); and PSF (26 patients; 36 levels). The authors demonstrated that the operative level lordosis change in all three interbody fusion procedures (LLIF, ALIF and TLIF) produced significantly greater segmental lordosis change compared to the traditional ‘on-lay’ PSF technique. These results are intuitive; however they serve to highlight the importance of interbody structural support in terms of lordosis restoration. Secondly, the authors reported no significant changes in supra-adjacent and subjacent level lordosis in all approaches except ALIF, where a decrease in supra-adjacent level lordosis was observed. Mean anterior and posterior disc heights increased in all approaches except PSF. The authors concluded that LLIF has the ability to improve sagittal contour as well as other interbody approaches and is superior to posterior approaches in disc height restoration (51). ALIF provided the greatest amount of segmental and overall lumbar lordosis correction. It must be acknowledged that this is a level III study and future comparative studies are required before any firm conclusions can be made.
ALIF vs. LLIF and TLIF
A study by Watkins et al. (52) of 220 consecutive patients with 309 operative levels were compared by surgery type: ALIF (184 levels), LLIF (86 levels), and TLIF (39 levels), with an average follow-up of 19.2 months in terms of sagittal correction and spondylolisthesis reduction. They concluded that improvement of lordosis was significant for both the ALIF and LLIF groups, but not the TLIF group. Intergroup analysis showed the ALIF group had significantly improved lordosis compared to both the other groups. The ALIF and LLIF groups had significantly increased disc height compared to the TLIF group. All the three groups significantly reduced spondylolisthesis, with no difference between the groups (52).
Part 2. Comparative evidence based on indication
Discogenic low back pain
There is limited comparative evidence for fusion approaches in the context of discogenic low back pain. The comparative evidence mostly investigates surgical fusion versus nonoperative management for discogenic low back pain. Bydon et al. (53) performed a systematic review and meta-analysis of five randomized trials including 523 lumbar fusion patients and 134 patients managed conservatively. Despite the significant improvement in ODI in the lumbar fusion groups in three studies, pooled data revealed no significant difference when compared with the non-operative group. It is not clear whether the pooled ODI difference of 7.39 points would have any clinical significance. As such, there remains limited comparative evidence for fusion approaches when the indication is discogenic low back pain. Many surgeons, however, prefer the ALIF approach for the management of discogenic low back pain, especially at L5/S1 and L4/5 as this technique spares the paraspinal and psoas muscles from iatrogenic injury, and provides efficient disc space clearance, therefore removing the annular pain generating fibers and achieving high fusion (54). TLIF is also thought to be a safe and effective technique in patients with intractable chronic lumbar discogenic pain with an acceptable overall work resumption rate based on a study by Takahashi et al. (55).
Isthmic spondylolisthesis
The most appropriate approach for the surgical treatment of isthmic spondylolisthesis remains a topic of controversy and debate. The comparative literature on this topic has predominantly compared PLIF vs. PLF approaches for low-grade adult isthmic spondylolisthesis. A prospective study with over 7-year follow-up by Cunningham et al. (56) demonstrated significantly higher Low Back Outcome Scores, PCS/MCS, SF-36 scores in the PLIF group compared to PLF. However, a randomized controlled trial comparing 39 PLF and 42 PLIF patients did not show any significant differences between the groups in terms of fusion rates, postoperative pain and functional outcomes, or complication rates (57). To address conflicting literature data, a meta-analysis by Luo et al. (58) pooled data from nine selected studies. PLF was shown to have lower fusion rates compared to PLIF (P=0.005, OR=0.29) and shorter operation duration. However, there was no difference detected in terms of postoperative VAS leg and back scores, blood loss, complication rates or ODI scores.
Kim et al. (39) compared outcomes of ALIF versus TLIF for adult low-grade isthmic spondylolisthesis at L4/5 and L5/S1 levels. TLIF had significantly higher ODI scores compared to ALIF at the L4/5 level. However, their radiographic results demonstrated that ALIF was superior in restoring disc height and whole lumbar lordosis at L5/S1 level. No differences in fusion rates were found between ALIF and TLIF. ALIF with PLF versus ALIF with percutaneous pedicle screw fixation has also been compared in elderly patients with L5/S1 isthmic spondylolisthesis (59). The authors noted a lower rate of fusion in patients with ALIF and percutaneous pedicle screw fixation compared to PLF. However, no significant differences in complication rates or VAS outcomes were noted between the groups. The retrospective nature of this study is a limitation that should be acknowledged; further well-designed studies are necessary for a more rigorous evaluation of these approaches.
Degenerative spondylolisthesis
Degenerative lumbar spondylolisthesis is characterized by facet joint degeneration, anterolisthesis and loss of disc height at affected levels, which can lead to central, lateral recess and foraminal stenosis (60,61). There has been a significant evolution in the treatment of this disease entity, with several landmark papers that have directed our treatment including the Spine Patient Outcomes Research Trial (SPORT), which offers the highest quality evidence to date (62). Although few would argue that the fusion of the diseased segment appears to offer the best and most durable results, treatment of this disease is best tailored to the individual. A study published in 2015 compared outcomes of 39 PLIF patients matched with 39 LLIF patients at levels L3/4 and L4/5 (63). This study demonstrated significantly better restoration of disc height, foraminal height and segmental lumbar lordosis in the LLIF group compared to PLIF, with similar operation times and lower average blood loss in the former approach. No differences in functional outcomes were reported. Another randomized study (64) compared TLIF versus uninstrumented PLF in 135 patients with degenerative lumbar spine disease with follow-up at 12 and 24 months. At follow-up, TLIF had a significantly higher proportion of patients who scored better in the global assessment measurement used, which the authors attributed to higher reoperation rates in the PLF group. Another study by Liu et al. (65) reached similar conclusions; they compared 101 TLIF vs. 125 PLIF cases for degenerative lumbar spondylolisthesis. PLIF was found to be associated with higher rates of complications such as nerve root injury, dural tears, blood transfusions, and increased operative duration and reoperation rates. Likewise, Fujimori et al. (66) demonstrated superiority for the TLIF approach compared to PLF in reducing listhesis and restoring disc height. However, no differences in ODI or PCS were statistically obtained. Whilst the comparative evidence for various techniques for lumbar degenerative spondylolisthesis remains limited, there is some data to suggest that lateral and transforaminal fusion approaches may result in lesser complications compared to posterior fusion approaches such as PLF and PLIF.
Degenerative scoliosis/kypho-scoliosis
Degenerative spinal deformity affects a significant portion of the elderly and is increasing in prevalence. It has been suggested that sagittal plane malalignment is central to the mechanisms of pain and disability in this population and has led to a significant shift toward a more evidence-based management paradigm (67). It results from cumulative degenerative changes focused in the intervertebral discs and facet joints that occur asymmetrically to produce deformity. Operative management, in carefully selected patients, yields satisfactory outcomes that appear to be superior to non-operative strategies, however these interventions are technically demanding for the surgeon, and medically stressful for the patient. Surgery aims to restore global spinal alignment, decompress neural elements, and achieve fusion with minimal complications.
There is evidence for idiopathic scoliosis that favors the anterior approach, with Nohara et al. reporting surgical outcomes of anterior fusion versus posterior fusion in patients with AIS Lenke type 1 and 2 over 10-year follow-up (68), however degenerative scoliosis is a very separate pathology. In this study, correction of scoliosis was found to be significantly improved in the anterior approach cohort compared to posterior cohort immediately after operation. However, greater loss of correction was found with the anterior approach group at 10-year follow-up. A study by Etemadifar et al. reported outcomes of using anterior-posterior fusion versus posterior alone for correction of Scheuermann’s kyphosis (69). Whilst clinical and radiology parameters including Cobb’s kyphosis angle, correction rate and correction loss were similar between the groups, complication rates, operation time and blood loss were found to be higher in the hybrid anterior-posterior fusion procedure.
More recently, MIS approaches have been compared with open approaches for idiopathic and degenerative scoliosis. Sarwahi et al. (70) reported in a study with 2-year follow-up that whilst MIS technique was feasible, there were no clear advances compared to open approaches in the context of idiopathic scoliosis, although this data did not translate to degenerative pathologies. For degenerative lumbar scoliosis, a recent meta-analysis of 12 studies (71) demonstrated significantly greater sagittal and coronal correction in patients receiving LLIF compared to the PLF and decompression-only cohort. Larger Cobb angles, greater sagittal balance correction, and higher reoperation rates were found in studies reporting the use of open fusion with osteotomy.
The literature remains heterogeneous in terms of techniques with a wide variety of options reported for lumbar degenerative scoliosis. Zhu et al. (72) reported on selective segmental transforaminal interbody fusion combined with posterior-instrumented spinal fusion, with ‘reasonable’ long-term clinical and radiographic outcomes. Manwaring et al. reported LLIF with Anterior Column Release (73), with the ability to powerfully restore lumbar lordosis and correct sagittal imbalance. This segmental MIS technique boasts equivalence to Smith Peterson Osteotomy correction of global radiographic parameters while simultaneously creating additional disc height and correcting coronal imbalance. If corrective surgery combining anterior and posterior approaches for adult degenerative scoliosis is contemplated, the timing of such interventions requires investigation. Staging the two procedures by 21 or more days apart decreases total perioperative transfusion requirements and significantly improves functional outcomes (74).
Adjacent segment degeneration
There are few papers reporting outcomes of surgery for adjacent segment degeneration. Whilst studies have been identified for treatment of adjacent segment degeneration with decompression alone, decompression and fusion, or decompression and disc arthroplasty, no comparative study has been identified to date (75). Miwa et al. retrospectively analyzed 18 patients who underwent repeat PLIF for adjacent segment disease, with 8 patients (44%) deteriorating again because of recurrence (76). There is some data for outcomes for cervical degenerative pathologies, with Bin et al. reporting the outcomes of ACDA for the treatment of adjacent segment disease in 32 patients, in which the authors suggested that ACDA was an effective treatment for post-ACDF adjacent segment disease (77), though no similar lumbar data exists.
Lumbar non-union
There is a paucity of data that assists the surgeon in making decisions on management strategies for lumbar non-union, and whether the non-union is best managed via the same approach as the initial surgery. Recent studies (22,24) have recommended posterior surgery (PLIF or TLIF) is well managed with high fusion rates via the anterior corridor (ALIF). Intuitively this makes sense as an unencumbered corridor is used with thorough endplate preparation to prepare an ample surface area to assist with revision fusion. Re-approaching the non-union segment via the initial exposure may involve extending the operative field with further morbidity especially with nerve root injury and CSF leak to the patient. There is no data except for a case report on both LLIF and OLIF (36) on revision strategies for posterior non-union. The converse also applies that if a patient has a non-union via an anterior technique (ALIF, LLIF, OLIF), that posterior approaches may represent a reasonable strategy as re-exposure of the anterior corridor can be high risk for vascular, bowel and ureter injury and should not be performed without vascular surgeon assistance (22,24).
Discussion
There is a large volume of data in the literature detailing clinical and radiological outcomes following specific interbody surgical interventions, however little robust class 1 or 2 clinical and biomechanical data comparing the various available techniques. Surgeons who have been trained in one specific interbody technique will have a tendency to favor that technique, irrespective of the pathology being treated, or the number of operative levels performed. The literature uniformly supports the concept of interbody techniques over on-lay posterior spinal fusion for sagittal and coronal plane deformities (78), with deformity reduction correlating with positive clinical outcomes in a number of well-performed studies (79). As spine surgery evolves to become its own subspecialty as it has in many parts of the world, surgical trainees will increasingly be exposed and trained in a variety of interbody techniques so that the surgeon is empowered with multiple options to manage difficult pathologies in an evidence-based fashion.
In terms of anterior versus posterior procedures, the available literature focuses on ALIF and TLIF as comparator techniques (14). In this systematic review, we established that blood loss and operative time in TLIF were less than in ALIF. These studies, however, described ALIF as a technique associated with an anterior and posterior combined procedure and TLIF as a single posterior procedure, and this may explain the difference in blood loss and operative time. However, there was no significant difference in the complication rate between ALIF and TLIF. No significant difference was found in the complications such as hematoma, wound infection, wound dehiscence, pedicle screw malposition, CSF leakage, and non-fusion between ALIF and TLIF. Some complications, such as incisional hernia, retrograde ejaculation, and venous injury were specific to ALIF. In addition, the risk of deep venous thrombosis and pulmonary embolus was increased after ALIF, and this may be related to the retraction of large vessels during the operation. Therefore, measures should be taken to prevent these complications, such as intraoperative anticoagulation (80) and vascular assistance with the ALIF approach and exposure (19,24). With further research comparing the stand-alone ALIF technique to TLIF, it is likely that the complication profile will alter in favor of ALIF as posterior stabilization is usually not necessary using contemporary ALIF cages with separate or integrated plate-screw fixation (14,19,22-24).
Sagittal balance and lumbar lordosis correction have become important goals as these indicators have been shown to significantly improve outcomes (81). LLIF has evolved to be a powerful MIS approach to lumbar spine fusion to achieve these goals. Recent studies now confirm that the operative level lordosis improves in all three interbody fusion procedures (LLIF, ALIF and TLIF) producing significantly greater segmental lordosis change compared to Posterior Spinal Fusion. However one study states that TLIF does not translate to measurable differences in lordosis restoration (52). As noted above, these results are intuitive and serve to highlight the importance of interbody structural support in terms of deformity reduction (51). Further studies on TLIF performed via a unilateral complete facetectomy demonstrate no significant improvement in segmental lordosis at the operative level (52). Watkins found an insignificant 0.8° change after TLIF (52). Hsieh et al. likewise reported no statistically significant difference in segmental lordosis after TLIF (13). They speculated that the difficulty in positioning the interbody device in the ideal anterior position, and the presence of a retained contralateral facet hinders posterior compression. The importance of positioning the cage as anterior as possible was highlighted by Kida et al. who reported significant improvement in segmental lordosis after cantilever TLIF (82).
In theory, ALIF allows re-expansion of the disc space and restoration of lumbar lordosis (17,83,84). The restoration of local and regional lordosis in the lumbar spine is critical for preserving and ideally improving spinal sagittal balance. The results of Hsieh et al. (13) indicated that ALIF was superior to TLIF in restoring the local disc angle and lumbar lordosis. ALIF increased the local disc angle by 8.3° and lumbar lordosis by 6.2°, whereas TLIF decreased the local disc angle by 0.1° and lumbar lordosis by 2.1°. Similarly, Kim et al. (85) reported that the change of disc height, segmental lordosis, and lumbar lordosis before and after surgery in ALIF was greater than in TLIF. These suggest that ALIF is superior to TLIF in its capacity to restore disc height and lumbar lordosis.
Regarding clinical outcomes, most studies (85,86) showed that clinical outcomes in ALIF were similar to TLIF. However, Kim et al. (39) report that the ODI score in TLIF is better than that in ALIF at L4-5, but not L5-S1, and they recommend that instrumented mini-TLIF is preferable at the L4-5 level, whereas instrumented mini-ALIF may be preferable at the L5-S1 level for the treatment of unstable isthmic spondylolisthesis. Different levels and pathologies may affect clinical outcomes of ALIF and TLIF, and further studies are awaited.
Our systematic review showed that comparable fusion rates between ALIF and TLIF approaches. ALIF was associated with restoration of disc height, segmental lordosis and total lumbar lordosis, lower dural injury but higher blood vessel injury (14). Regarding financial costs, there has been conflicting evidence in the literature, with some studies reporting ALIF to be cheaper (19) and other studies reporting it to be more expensive (87) than surgical treatment with TLIF.
A further question that warrants discussion is the role of multiple approaches being performed on the same day versus staging of procedures. It is reported that the timing of surgical staging has a significant impact on the complications and functional outcomes of adult spinal deformity surgery. Hassanzadeh et al. (88) reported that for patients who require both anterior and posterior surgery for spinal deformity correction, staging the two procedures 21 or more days apart decreases total perioperative transfusion requirements and significantly improves functional outcomes. However, there is no available data on single level anterior/posterior interventions performed as same -day surgery versus delayed surgeries.
There are multiple limitations in this systematic review (14). Some studies report ALIF and TLIF combined with posterolateral fusion, therefore skewing the potential fusion results and outcomes. In addition, studies reveal a heterogeneous patient population, with different levels and pathologies reported that impact radiological fusion rates and clinical outcomes. Therefore, the conclusions cannot be made about the effects of different levels and pathologies on clinical outcomes. ALIF, TLIF and PLIF remain the more commonly performed techniques for lumbar interbody fusion (LIF). LLIF has established its place as a robust technique for deformity correction and interbody fusion, with OLIF requiring further studies and data to establish its place.
Available data suggests that anterior techniques are superior to posterior in terms of disc height restoration, lumbar lordosis and deformity correction, and that clinical outcomes and fusion rates were similar to those in posterior techniques. This data, however, is based on heterogeneous studies with multiple indications and through comparison is difficult to make.
The authors provide the following recommendations for future studies based on the available literature:
- Clinical and radiological outcomes on particular indications performed via specific approaches at specific levels. Is ALIF, LLIF, TLIF or PLIF the clinically superior technique for degenerative spondylolisthesis at L4/5? Assuming one technique is superior radiologically, does this translate to clinical superiority?
- What is the superior option for the management of degenerative lumbar and thoracolumbar scoliosis? There are numerous options here including posterior only, lateral and anterior or a combination of all 3 approaches. Further questions remain as to precise timing of such interventions, and if various approaches should be performed on the same day or as a staged procedure;
- Revision strategies for failed lumbar fusions. Is a failed fusion/non-union best managed via the same approach as the initial surgery or via a new corridor?
- Adjacent segment degeneration. Should a revision be performed via the same approach/corridor as the index surgery, or are radiological and clinical results superior if a new corridor of approach is used?
Recommendations
Based on the available evidence in the literature, the following recommendations and guidelines are proposed, based on the level of pathology and indication (Table 1).
Table 1
| Option/level | L5/S1 | L4/5 | L3/4 | L2/3 | T12/L1/2 |
|---|---|---|---|---|---|
| PLIF | +++ | +++ | +++ | +++ | ++ |
| TLIF | +++ | +++ | +++ | +++ | ++ |
| ALIF | +++ | +++ | + | – | – |
| LLIF | – | +++ | +++ | +++ | ++ |
| OLIF/ATP | ++ | +++ | +++ | +++ | + |
+, fair option; ++, good option; +++, excellent option; -, poor option/not suitable. PLIF, posterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion; ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; OLIF, oblique lumbar interbody fusion; ATP, anterior to psoas.
L5/S1
- ALIF preferred method for discogenic LBP and lordosis restoration;
- PLIF and TLIF reasonable methods especially with central canal stenosis or recurrent disc herniation (89);
- PLIF preferred approach for Isthmic spondylolisthesis due to the high risk of L5 nerve injury (90);
- OLIF early data is promising; LLIF not suitable.
L4/5
- PLIF and TLIF good option for degenerative spondylolisthesis;
- ALIF/OLIF/LLIF all reasonable options for lordosis and deformity correction, however increased risk of lumbar plexus/psoas injury with LLIF.
L2/3 and L3/4
- All techniques except for ALIF suitable;
- ALIF not recommended as high risk; need experienced vascular surgical assistance;
- For deformity correction, antero-lateral techniques preferred including LLIF/OLIF.
T12/L1 and L1/2
- ALIF not suitable;
- PLIF/TLIF difficult if cord or conus inhibits dural retraction for insertion of implant/s;
- LLIF good option especially for deformity correction.
Multilevel
- Multilevel usually involves deformity correction if performed for degenerative pathologies;
- Combination of anterior/lateral and posterior techniques provides powerful deformity correction;
- Multilevel ALIF/LLIF/OLIF provide sagittal and coronal correction but may require supplemental posterior fixation.
Conclusions
Interbody fusion remains an effective treatment option for a range of spinal disorders including degenerative pathologies, trauma, infection and neoplasia. There are various approaches available for interbody fusion including PLIF, TLIF, MI-TLIF, OLIF/ATP, ALIF and LLIF. There is limited comparative evidence demonstrating that one approach is superior to another in terms of fusion or clinical outcomes. The present study provides a broad overview of the advantages, disadvantages, indications and evidence for different LIF techniques. We propose a set of recommendations that may assist with deciding the most appropriate surgical approach for the patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine 2005;2:670-2. [PubMed]
- Eck JC, Hodges S, Humphreys SC. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg 2007;15:321-9. [PubMed]
- Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci 2012;19:829-35. [PubMed]
- Phan K, Rao PJ, Scherman DB, et al. Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J Clin Neurosci 2015;22:1714-21. [PubMed]
- Briggs H, Milligan PR. Chip fusion of the low back following exploration of the spinal canal. J Bone Joint Surg Am 1944;26:125-30.
- Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author's transl). Z Orthop Ihre Grenzgeb 1982;120:343-7. [PubMed]
- Lestini WF, Fulghum JS, Whitehurst LA. Lumbar spinal fusion: advantages of posterior lumbar interbody fusion. Surg Technol Int 1994;3:577-90. [PubMed]
- Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009;2:118-26. [PubMed]
- Fan SW, Hu ZJ, Fang XQ, et al. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg 2010;2:194-200. [PubMed]
- Park J, Kim Y, Hong H, et al. Comparison between posterior and transforaminal approaches for lumbar interbody fusion. J Korean Neurosurg Soc 2005;37:340-4.
- Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976) 2001;26:567-71. [PubMed]
- Zhang Q, Yuan Z, Zhou M, et al. A comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion: a literature review and meta-analysis. BMC Musculoskelet Disord 2014;15:367. [PubMed]
- Hsieh PC, Koski TR, O'Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 2007;7:379-86. [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion - systematic review and meta-analysis. Br J Neurosurg 2015;705-11. [PubMed]
- Audat Z, Moutasem O, Yousef K, et al. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J 2012;53:183-7. [PubMed]
- Sakeb N, Ahsan K. Comparison of the early results of transforaminal lumbar interbody fusion and posterior lumbar interbody fusion in symptomatic lumbar instability. Indian J Orthop 2013;47:255-63. [PubMed]
- McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976) 2005;30:S60-5. [PubMed]
- Rao PJ, Loganathan A, Yeung V, et al. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery 2015;76:7-23; discussion 23-4. [PubMed]
- Malham GM, Parker RM, Ellis NJ, et al. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine 2014;21:851-60. [PubMed]
- Phan K, Mobbs RJ. Sacrum fracture following L5-S1 stand-alone interbody fusion for isthmic spondylolisthesis. J Clin Neurosci 2015;22:1837-9. [PubMed]
- Rao PJ, Ghent F, Phan K, et al. Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci 2015;22:1619-24. [PubMed]
- Mobbs RJ, Phan K, Thayaparan GK, et al. Anterior Lumbar Interbody Fusion as a Salvage Technique for Pseudarthrosis following Posterior Lumbar Fusion Surgery. Global Spine J 2015. Available online: http://www.researchgate.net/publication/278031570_Anterior_Lumbar_Interbody_Fusion_as_a_Salvage_Technique_for_Pseudarthrosis_following_Posterior_Lumbar_Fusion_Surgery
- Rao PJ, Maharaj MM, Phan K, et al. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J 2015;15:817-24. [PubMed]
- Mobbs RJ, Phan K, Daly D, et al. Approach-Related Complications of Anterior Lumbar Interbody Fusion: Results of a Combined Spine and Vascular Surgical Team. Global Spine J 2015. Available online: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0035-1557141
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [PubMed]
- Arnold PM, Anderson KK, McGuire RA Jr. The lateral transpsoas approach to the lumbar and thoracic spine: A review. Surg Neurol Int 2012;3:S198-215. [PubMed]
- Malham GM, Parker RM, Goss B, et al. Clinical results and limitations of indirect decompression in spinal stenosis with laterally implanted interbody cages: results from a prospective cohort study. Eur Spine J 2015;24:339-45. [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Maintenance of Segmental Lordosis and Disc Height in Standalone and Instrumented Extreme Lateral Interbody Fusion (XLIF). J Spinal Disord Tech 2014; [Epub ahead of print]. [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012;2012:246989.
- Barbagallo GM, Albanese V, Raich AL, et al. Lumbar Lateral Interbody Fusion (LLIF): Comparative Effectiveness and Safety versus PLIF/TLIF and Predictive Factors Affecting LLIF Outcome. Evid Based Spine Care J 2014;5:28-37. [PubMed]
- Lee YS, Park SW, Kim YB. Direct lateral lumbar interbody fusion: clinical and radiological outcomes. J Korean Neurosurg Soc 2014;55:248-54. [PubMed]
- Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22:691-9; discussion 700. [PubMed]
- Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J 2012;6:89-97. [PubMed]
- Ohtori S, Orita S, Yamauchi K, et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Lumbar Spinal Degeneration Disease. Yonsei Med J 2015;56:1051-9. [PubMed]
- Ohtori S, Mannoji C, Orita S, et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Degenerated Lumbar Spinal Kyphoscoliosis. Asian Spine J 2015;9:565-72. [PubMed]
- Phan K, Mobbs RJ. Oblique lumbar interbody fusion for revision of non-union following prior posterior surgery: a case report. Orthopaedic Surgery 2015; [Epub ahead of print]. [PubMed]
- Hacker RJ. Comparison of interbody fusion approaches for disabling low back pain. Spine (Phila Pa 1976) 1997;22:660-5; discussion 665-6. [PubMed]
- Faundez AA, Mehbod AA, Wu C, et al. Position of interbody spacer in transforaminal lumbar interbody fusion: effect on 3-dimensional stability and sagittal lumbar contour. J Spinal Disord Tech 2008;21:175-80. [PubMed]
- Kim JS, Lee KY, Lee SH, et al. Which lumbar interbody fusion technique is better in terms of level for the treatment of unstable isthmic spondylolisthesis? J Neurosurg Spine 2010;12:171-7. [PubMed]
- Dorward IG, Lenke LG, Bridwell KH, et al. Transforaminal versus anterior lumbar interbody fusion in long deformity constructs: a matched cohort analysis. Spine (Phila Pa 1976) 2013;38:E755-62. [PubMed]
- Gjessing MH. Osteoplastic anterior fusion of the lower lumbar spine in spondylolisthesis, localized spondylosis, and tuberculous spondylitis. Acta Orthop Scand 1951;20:200-13. [PubMed]
- Cloward RB. Spondylolisthesis: treatment by laminectomy and posterior interbody fusion. Clin Orthop Relat Res 1981;74-82. [PubMed]
- Schnee CL, Freese A, Ansell LV. Outcome analysis for adults with spondylolisthesis treated with posterolateral fusion and transpedicular screw fixation. J Neurosurg 1997;86:56-63. [PubMed]
- Salehi SA, Tawk R, Ganju A, et al. Transforaminal lumbar interbody fusion: surgical technique and results in 24 patients. Neurosurgery 2004;54:368-74; discussion 374. [PubMed]
- Cloward RB. The treatment of ruptured lumbar intervertebral disc by vertebral body fusion. III. Method of use of banked bone. Ann Surg 1952;136:987-92. [PubMed]
- Lin PM. A technical modification of Cloward's posterior lumbar interbody fusion. Neurosurgery 1977;1:118-24. [PubMed]
- Yan DL, Li J, Gao LB, et al. Comparative study on two different methods of lumbar interbody fusion with pedicle screw fixation for the treatment of spondylolisthesis. Zhonghua Wai Ke Za Zhi 2008;46:497-500. [PubMed]
- Zhuo X, Hu J, Li B, et al. Comparative study of treating recurrent lumbar disc protrusion by three different surgical procedures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009;23:1422-6. [PubMed]
- Mobbs RJ, Sivabalan P, Li J, et al. Hybrid technique for posterior lumbar interbody fusion: a combination of open decompression and percutaneous pedicle screw fixation. Orthop Surg 2013;5:135-41. [PubMed]
- Mobbs RJ, Sivabalan P, Li J. Technique, challenges and indications for percutaneous pedicle screw fixation. J Clin Neurosci 2011;18:741-9. [PubMed]
- Sembrano JN, Yson SC, Horazdovsky RD, et al. Radiographic Comparison of Lateral Lumbar Interbody Fusion Versus Traditional Fusion Approaches: Analysis of Sagittal Contour Change. Int J Spine Surg 2015;9:16. [PubMed]
- Watkins RG 4th, Hanna R, Chang D, et al. Sagittal alignment after lumbar interbody fusion: comparing anterior, lateral, and transforaminal approaches. J Spinal Disord Tech 2014;27:253-6. [PubMed]
- Bydon M, De la Garza-Ramos R, Macki M, et al. Lumbar fusion versus nonoperative management for treatment of discogenic low back pain: a systematic review and meta-analysis of randomized controlled trials. J Spinal Disord Tech 2014;27:297-304. [PubMed]
- Mobbs RJ, Loganathan A, Yeung V, et al. Indications for anterior lumbar interbody fusion. Orthop Surg 2013;5:153-63. [PubMed]
- Takahashi T, Hanakita J, Minami M, et al. Surgical outcome and postoperative work status of lumbar discogenic pain following transforaminal interbody fusion. Neurol Med Chir (Tokyo) 2011;51:101-7. [PubMed]
- Cunningham JE, Elling EM, Milton AH, et al. What is the optimum fusion technique for adult isthmic spondylolisthesis--PLIF or PLF? A long-term prospective cohort comparison study. J Spinal Disord Tech 2013;26:260-7. [PubMed]
- Lee GW, Lee SM, Ahn MW, et al. Comparison of posterolateral lumbar fusion and posterior lumbar interbody fusion for patients younger than 60 years with isthmic spondylolisthesis. Spine (Phila Pa 1976) 2014;39:E1475-80. [PubMed]
- Luo J, Cao K, Yu T, et al. Comparison of Posterior Lumbar Interbody Fusion versus Posterolateral Fusion for the Treatment of Isthmic Spondylolithesis. J Spinal Disord Tech 2015; [Epub ahead of print]. [PubMed]
- Shim JH, Kim WS, Kim JH, et al. Comparison of instrumented posterolateral fusion versus percutaneous pedicle screw fixation combined with anterior lumbar interbody fusion in elderly patients with L5-S1 isthmic spondylolisthesis and foraminal stenosis. J Neurosurg Spine 2011;15:311-9. [PubMed]
- Herkowitz HN. Degenerative lumbar spondylolisthesis: evolution of surgical management. Spine J 2009;9:605-6. [PubMed]
- Martin CR, Gruszczynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine (Phila Pa 1976) 2007;32:1791-8. [PubMed]
- Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;91:1295-304. [PubMed]
- Pawar AY, Hughes AP, Sama AA, et al. A Comparative Study of Lateral Lumbar Interbody Fusion and Posterior Lumbar Interbody Fusion in Degenerative Lumbar Spondylolisthesis. Asian Spine J 2015;9:668-74. [PubMed]
- Jalalpour K, Neumann P, Johansson C, et al. A Randomized Controlled Trial Comparing Transforaminal Lumbar Interbody Fusion and Uninstrumented Posterolateral Fusion in the Degenerative Lumbar Spine. Global Spine J 2015;5:322-8. [PubMed]
- Liu J, Deng H, Long X, et al. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J 2015; [Epub ahead of print]. [PubMed]
- Fujimori T, Le H, Schairer WW, et al. Does Transforaminal Lumbar Interbody Fusion Have Advantages over Posterolateral Lumbar Fusion for Degenerative Spondylolisthesis? Global Spine J 2015;5:102-9. [PubMed]
- Ailon T, Smith JS, Shaffrey CI, et al. Degenerative Spinal Deformity. Neurosurgery 2015;77:S75-91. [PubMed]
- Nohara A, Kawakami N, Saito T, et al. Comparison of Surgical Outcomes Between Anterior Fusion and Posterior Fusion in Patients With AIS Lenke Type 1 or 2 that Underwent Selective Thoracic Fusion -Long-term Follow-up Study Longer Than 10 Postoperative Years. Spine (Phila Pa 1976) 2015;40:1681-9. [PubMed]
- Etemadifar M, Ebrahimzadeh A, Hadi A, et al. Comparison of Scheuermann's kyphosis correction by combined anterior-posterior fusion versus posterior-only procedure. Eur Spine J 2015; [Epub ahead of print]. [PubMed]
- Sarwahi V, Horn JJ, Kulkarni PM, et al. Minimally Invasive Surgery in Patients with Adolescent Idiopathic Scoliosis: Is it Better than the Standard Approach? A Two Year Follow-Up Study. J Spinal Disord Tech 2014; [Epub ahead of print]. [PubMed]
- Dangelmajer S, Zadnik PL, Rodriguez ST, et al. Minimally invasive spine surgery for adult degenerative lumbar scoliosis. Neurosurg Focus 2014;36:E7. [PubMed]
- Zhu Y, Liu HY, Wang B, et al. Long-term clinical outcomes of selective segmental transforaminal lumbar interbody fusion and posterior spinal fusion for degenerative lumbar scoliosis. Zhonghua Yi Xue Za Zhi 2013;93:3577-81. [PubMed]
- Manwaring JC, Bach K, Ahmadian AA, et al. Management of sagittal balance in adult spinal deformity with minimally invasive anterolateral lumbar interbody fusion: a preliminary radiographic study. J Neurosurg Spine 2014;20:515-22. [PubMed]
- Hassanzadeh H, Gjolaj JP, El Dafrawy MH, et al. The timing of surgical staging has a significant impact on the complications and functional outcomes of adult spinal deformity surgery. Spine J 2013;13:1717-22. [PubMed]
- Chou D, Dekutoski M, Hermsmeyer J, et al. The treatment of lumbar adjacent segment pathology after a previous lumbar surgery: a systematic review. Spine (Phila Pa 1976) 2012;37:S180-8. [PubMed]
- Miwa T, Sakaura H, Yamashita T, et al. Surgical outcomes of additional posterior lumbar interbody fusion for adjacent segment disease after single-level posterior lumbar interbody fusion. Eur Spine J 2013;22:2864-8. [PubMed]
- Bin S, Xiangwang H, Sheng X, et al. Artificial Cervical Disc Replacement for the Treatment of Adjacent Segment Disease after Anterior Cervical Decompression and Fusion. J Spinal Disord Tech 2014; [Epub ahead of print]. [PubMed]
- Kowalski RJ, Ferrara LA, Benzel EC. Biomechanics of bone fusion. Neurosurg Focus 2001;10:E2. [PubMed]
- Dorward IG, Lenke LG, Stoker GE, et al. Radiographic and Clinical Outcomes of Posterior Column Osteotomies in Spinal Deformity Correction. Spine (Phila Pa 1976) 2014; [Epub ahead of print]. [PubMed]
- Sim EM, Claydon MH, Parker RM, et al. Brief intraoperative heparinization and blood loss in anterior lumbar spine surgery. J Neurosurg Spine 2015;23:309-13. [PubMed]
- Glassman SD, Berven S, Bridwell K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 2005;30:682-8. [PubMed]
- Kida K, Tadokoro N, Kumon M, et al. Can cantilever transforaminal lumbar interbody fusion (C-TLIF) maintain segmental lordosis for degenerative spondylolisthesis on a long-term basis? Arch Orthop Trauma Surg 2014;134:311-5. [PubMed]
- Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 2002;15:337-49. [PubMed]
- Pavlov PW, Meijers H, van Limbeek J, et al. Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation. Spine (Phila Pa 1976) 2004;29:1893-9; discussion 1900.
- Kim JS, Kang BU, Lee SH, et al. Mini-transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation: a comparison of surgical outcomes in adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech 2009;22:114-21. [PubMed]
- Faundez AA, Schwender JD, Safriel Y, et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J 2009;18:203-11. [PubMed]
- Goz V, Weinreb JH, Schwab F, et al. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J 2014;14:2019-27. [PubMed]
- Hassanzadeh H, Jain A, El Dafrawy MH, et al. Three-column osteotomies in the treatment of spinal deformity in adult patients 60 years old and older: outcome and complications. Spine (Phila Pa 1976) 2013;38:726-31. [PubMed]
- Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 8: lumbar fusion for disc herniation and radiculopathy. J Neurosurg Spine 2005;2:673-8. [PubMed]
- Jacobs WC, Vreeling A, De Kleuver M. Fusion for low-grade adult isthmic spondylolisthesis: a systematic review of the literature. Eur Spine J 2006;15:391-402. [PubMed]


