Abstract
Introduction
Central sarcopenia as a surrogate for frailty has recently been studied as a predictor of outcome in elderly medical patients, but less is known about how this metric relates to outcomes after trauma. We hypothesized that psoas:lumbar vertebral index (PLVI), a measure of central sarcopenia, is associated with increased morbidity and mortality in elderly trauma patients.
Methods
A query of our institutional trauma registry from 2005 to 2010 was performed. Data was collected prospectively for the Pennsylvania Trauma Outcomes Study (PTOS). Inclusion criteria: age >55 years, ISS >15, and ICU LOS >48 h. Using admission CT scans, psoas:vertebral index was computed as the ratio between the mean cross-sectional areas of the psoas muscles and the L4 vertebral body at the level of the L4 pedicles. The 50th percentile of the psoas:L4 vertebral index value was determined, and patients were grouped into high (>0.84) and low (≤0.83) categories based on their relation to the cohort median. Primary endpoints were mortality and morbidity (as a combined endpoint for PTOS-defined complications). Univariate logistic regression was used to test the association between patient factors and mortality. Factors found to be associated with mortality at p < 0.1 were entered into a multivariable model.
Results
A total of 180 patients met the study criteria. Median age was 74 years (IQR 63–82), median ISS was 24 (IQR 18–29). Patients were 58 % male and 66 % Caucasian. Mean PLVI was 0.86 (SD 0.25) and was higher in male patients than female patients (0.91 ± 0.26 vs. 0.77 ± 0.21, p < 0.001). PLVI was not associated with mortality in univariate or multivariable modeling. After controlling for comorbidities, ISS, and admission SBP, low PLVI was found to be strongly associated with morbidity (OR 4.91, 95 % CI 2.28–10.60).
Conclusions
Psoas:lumbar vertebral index is independently and negatively associated with posttraumatic morbidity but not mortality in elderly, severely injured trauma patients. PLVI can be calculated quickly and easily and may help identify patients at increased risk of complications.
Similar content being viewed by others
Introduction
Due to population aging, the number of elderly patients presenting to trauma centers will continue to increase. Advanced age is a known risk factor for morbidity and mortality after traumatic injury [1, 2] but it is clear that other factors such as medical comorbidities also contribute strongly to outcomes [3, 4]. Recently, attention has been focused on the concept of frailty as a driver of outcomes across a wide range of disease states in the elderly, including chronic kidney disease, malignancy, and aortic valve disease [5–7]. The original description of frailty includes difficulty with activities of daily living, reduced speed of ambulation, subjective feelings of exhaustion, unintentional weight loss, and weakness as measured by grip strength [8]. The association of frailty with outcomes after trauma is not well characterized, perhaps in part because frailty evaluation using conventional metrics in the trauma population is not practicable. Trauma is by its nature unpredictable, and for this reason most injured patients will present without baseline measurements of frailty. Attempts to measure frailty after injury using conventional metrics may be limited by altered mental status or inability to participate in physical activity. An attractive solution to this problem is to measure a surrogate for frailty that does not rely upon active patient participation, such as radiographically determined central sarcopenia [9]. Central sarcopenia, as measured by psoas cross-sectional area, has been associated with poor outcomes after liver transplantation [10] and open repair of ruptured aortic aneurysms [11], but the impact of central sarcopenia has not yet been well characterized in the elderly trauma population.
We sought to evaluate whether central sarcopenia, as measured by psoas cross-sectional area on admission imaging, is associated with outcomes in elderly trauma patients. We hypothesized that lower psoas cross-sectional area would be associated with increased morbidity and mortality in a cohort of elderly, severely injured trauma patients.
Materials and methods
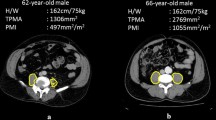
After obtaining institutional review board approval, a query of our institutional trauma registry across a 5-year time period (2005–2010) was performed. Data for our trauma registry are collected prospectively and reported to the Pennsylvania Trauma Outcomes Study (PTOS) database by trained nurse abstractors. Study inclusion criteria were age ≥55 years, severe injury (Injury Severity Score (ISS) >15), and ICU length of stay >48 h. Patients were excluded if they suffered a critical head injury (defined as a Head/Neck Abbreviated Injury Scale (AIS) score ≥5), did not receive admission cross-sectional imaging of the abdomen, had fractures or preexisting hardware of the 4th lumbar vertebral body, or had a retroperitoneal hematoma that distorted the cross-sectional area of the psoas at the level of the L4 vertebral body. Patient demographics (age, sex, and race), physiologic variables on presentation, mechanism of injury, Injury Severity Score, hospital length of stay (HLOS), ICU length of stay (ILOS), ventilator days, and comorbidities were abstracted from the institutional registry. Morbidity was measured by PTOS-defined occurrences (see “Appendix 1”). Patients meeting all inclusion criteria with no exclusion criteria were then uploaded into a RED Cap database [12]. Computed tomography (CT) studies of the abdomen were obtained from the medical record and evaluated for each patient by one of three trained reviewers (DG, LE, DH). Prior to abstracting the study CTs, a sample of studies were independently abstracted by the three reviewers and results were compared in order to assess for inter-rater reliability. For each study, the right and left psoas muscle cross-sectional areas (PCSA) were measured at the level of the L4 vertebral body immediately inferior to the origin of the posterior elements. To normalize for body habitus, the cross-sectional area of the L4 vertebral body was also recorded at this level (Fig. 1). Mean PSCA was calculated for each patient, and the ratio between mean PSCA and L4 vertebral body area was calculated using the following formula:
The 50th percentile of psoas:L4 vertebral index (PLVI) value was determined and patients were grouped into high (>0.84) and low (≤0.83) categories based on their relation to the cohort median. Univariate analyses of patient demographic variables, admission vital signs, comorbidities, and outcome measures between the two groups were performed.
Primary outcomes
The primary outcomes of interest of the study were in-hospital mortality and morbidity. Morbidity was defined as a composite endpoint of any of 37 PTOS-defined complications (see “Appendix 1”) and as subgroups of respiratory complications (acute respiratory distress syndrome, acute respiratory failure, aspiration/aspiration pneumonia, atelectasis, or pneumonia), infectious complications (sepsis, septicemia, acute sinusitis, soft tissue infection, urinary tract infection, pneumonia, wound infection, central nervous system infection, or empyema), organ failure (acute respiratory distress syndrome, coagulopathy, acute renal failure, or liver failure), thromboembolic complications (pulmonary embolism or deep venous thrombosis), and hemorrhagic complications (coagulopathy or postoperative hemorrhage).
Secondary outcomes of interest were hospital length of stay (HLOS), ICU length of stay, and total ventilator days, which were examined between groups. In addition, the PLVI category was examined via mechanism of injury.
Statistical analyses
The associations of baseline characteristics with morbidity and with hospital mortality were tested using t test for continuous normally distributed variables and Mann–Whitney or Kruskal–Wallis for continuous non-normally distributed variables. Categorical variables were compared using χ 2 or Fischer’s exact tests, as appropriate. Univariate logistic regression was used to assess the association between independent variables and the primary outcomes of interest.
Multivariable logistic regression models were developed to adjust the association of PLVI with morbidity and mortality for potential confounders. Variables found to be associated with the outcome of interest in univariate analysis with a p value of <0.10 were included in the multivariate logistic models. All statistical analyses were performed using SPSS version 19.0 (IBM, Chicago IL).
Secondary outcomes of interest were hospital length of stay (HLOS), ICU length of stay, and total ventilator days. In addition, mechanism injury by PLVI category was examined.
Results
At total of 234 patients met the inclusion criteria, of whom 54 were excluded (Fig. 2), leaving 180 patients for analysis. Median age in the cohort was 74 years (IQR 63–82), and median ISS was 24 (IQR 18–29). Patients were 58 % male and 66 % Caucasian.
There was no correlation between L4 vertebral body CSA and age, but psoas CSA did have a weak negative relationship with age (R 2 = 0.15). The correlation between right and left psoas CSA was high but not complete (R 2 = 0.75), so mean psoas CSA was used to calculate the PLVI. PLVI values were found to be normally distributed within the study population, with a mean value of 0.85 ± 0.25. PLVI values were higher in male patients than in female patients (0.91 ± 0.26 vs. 0.77 ± 0.21, p < 0.001). Lumbar–psoas vertebral index rating reliability was found to be good between raters, with a maximum deviation of 23 %, which resulted in no misclassifications based on groups. Compared to patients in the high-PLVI group, patients in the low-PLVI group were older (median age 79 (IQR 72–85) vs. 70 (IQR 60–77), p < 00.1), less likely to be male (43 vs. 71 %, p < 0.001), and less likely to be Caucasian (63 vs. 69 %, p = 0.04). No significant differences were seen between the two groups in terms of number of baseline comorbidities, mechanism of injury, ISS, or admission vitals (Table 1). In unadjusted analysis, patients in the low-PLVI group had higher morbidity (83 vs. 58 %, p < 0.001), but no difference was seen in mortality (16 vs. 10 %, p = 0.26) or any of the secondary endpoints of hospital length of stay, ICU length of stay, or ventilator days.
In univariate analysis (Table 2), PLVI category was not significantly associated with mortality. Only age in years (OR 1.05, 95 % CI 1.01–1.10), comorbidities (OR 1.30, 95 % CI 1.01–1.60), and AIS head (OR 1.44, 95 % CI 1.02–2.02) were found to be associated with mortality. In contrast, low PLVI was strongly associated with development of morbidity (OR 3.66, 95 % CI 1.83–7.32), as was ISS (OR 1.05, 95 % CI 1.01–1.10).
Multivariable logistic regression analysis (Table 3) did not demonstrate an association between PLVI and mortality, but even after controlling for baseline comorbidities, ISS, and admission SBP, low PLVI was found to be strongly associated with morbidity (OR 4.91, 95 % CI 2.28–10.60).
In the unadjusted analysis of morbidity subcategories of complications (Table 4), we found that infectious complications were statistically more likely to occur in the low PLVI group (60 vs. 39 %, p = 0.03). Mechanism of injury also differed between the two groups (Table 5), with patients in the low PLVI category more likely to have sustained a fall (59 vs. 43 %, p < 0.05) and less likely to have sustained an MVC (20 vs. 33 %, p < 0.05).
Discussion
With an increasing elderly population living with greater numbers of chronic medical conditions and decreased physiologic reserve, the concept of frailty is emerging as a significant syndrome in the elderly patient population. Frailty further diminishes the older patient’s ability to compensate when stressed. Fried and colleagues [8] described frailty as a phenotype that includes three of the following five characteristics: unintentional weight loss, slow walking speed, self-reported exhaustion, low physical activity, and weakness as measured by grip strength. The physiology of aging or an inherent decline in various organ systems accounts for this phenotype, and includes increased levels of inflammatory markers (CRP, IL-6), diminution in bone density (osteoporosis), cognitive changes (delirium and dementia), and sarcopenia [13].
In this cohort of elderly, severely injured trauma patients, we found that central sarcopenia as measured by PLVI was associated with morbidity but not mortality. The PLVI has several advantages over other metrics of frailty available for use in the trauma population. Because the majority of blunt trauma patients will undergo cross-sectional imaging upon admission [14], the ability to measure PLVI is present early on for most elderly trauma patients. Since imaging occurs extremely early in the hospital course of trauma patient, admission PLVI is unlikely to be greatly affected by injuries, interventions undertaken to treat injuries, or complications developing thereafter. The admission PLVI can therefore be thought of as a baseline “snapshot” that represents the patient’s condition at the time of injury. Because this metric is objective, does not require patient cooperation or recall, and does not require the ability to ambulate, it may be far easier to assess in patients suffering traumatic injury.
The use of central sarcopenia as a proxy for frailty and predictor of outcomes has been studied in other groups of surgical patients in recent years. Decreased psoas cross-sectional area has been shown to be associated with mortality after liver transplantation [10], elective abdominal aortic aneurysm repair [15], and esophagectomy [16]. In a cohort of patients undergoing hepatic resection for colorectal metastases, Peng et al. [17] found that a total psoas cross-sectional area of <500 mm was associated with increased risk of postoperative complications and hospital length of stay, but not mortality. Of note, our methodology differs from the above-referenced studies in that, rather than reporting the combined cross-sectional area of the psoas muscles at the L4 vertebra (or “total psoas area”, TPA), we report the ratio of psoas CSA to the L4 vertebral body CSA. The intent of this methodology is to provide a PSCA value that is normalized to individual patient body habitus, but further research will be necessary to determine the optimal measurement of central sarcopenia.
Injury Severity Score is one of the best known predictors of mortality in trauma patients, and so it may seem surprising that this failed to predict mortality in our models. However, the distribution of ISS in our cohort is relatively tightly distributed around the median of 24 (range 16–59), with 80 % of our cohort having an ISS between 16 and 32. Given that the predicted mortality in this range of ISS for patients greater than 55 years of age would be expected to be between ~5 and 13 %, it is not entirely surprising, considering the relatively small sample size, that no difference is seen in our model.
In the subgroup analysis, the only category of complications to reach statistical significance between the two groups was infectious complications. Frailty has been associated with alterations in both the innate and adaptive immune system [18–20], and it is thus possible that in the presence of frailty as reflected by low PLVI, host immune responses may be compromised. Alternatively, it may be that the frailty phenotype necessitates interventions which result in increased risk of infections. For instance, patients who are unable to be mobilized from bed may be subjected to prolonged use of indwelling urinary catheters, resulting in increased risk of urinary tract infection.
Despite our findings, this study has some important limitations. The utility of using “any complication” as a combined primary endpoint may be questioned, as different complications have different risk factors which may not be equally distributed between our two groups. Given our relatively small sample size and the degree of complexity that would be introduced in order to control for all of the risk factors for each individual complication, we have chosen to look at an aggregate measure instead. However, the use of morbidity as a combined endpoint may be more meaningful than rates of specific complications, as the aggregate reflects the overall baseline condition and host response to trauma. Additionally, as no specific treatments are available to protect frail patients from posttraumatic morbidity, the therapeutic benefit of a frailty index is currently very limited. However, the addition of central sarcopenia to the traditional predictors of outcome may help inform clinicians when discussing likely outcomes with family patients and their families. Future prospective studies may identify specific opportunities for improvement in this cohort. Finally, this study represents a single-institution experience with a limited number of patients, and as such may not be applicable to other centers. Specifically, the LPVI median of 0.84 from which we derive our cut-point to divide the cohort into “high” and “low” PLVI groups may not be valid in other populations. Further work with larger cohorts may help clarify this issue.
In conclusion, the PLVI is an easily-obtained metric which may help physicians caring for trauma patients to identify a subpopulation of elderly patients at increased risk of morbidity, independent of age and comorbidities. Further investigative efforts should be directed towards translating risk identification into risk reduction.
References
Tornetta Iii P, Mostafavi H, Riina J, Turen C, Reimer B, Levine R, et al. Morbidity and mortality in elderly trauma patients. J Trau Inj Infect Crit Care. 1999;46(4):702–6.
Labib N, Nouh T, Winocour S, Deckelbaum D, Banici L, Fata P, et al. Severely injured geriatric population: morbidity, mortality, and risk factors. J Trau Inj Infect Crit Care. 2011;71(6):1908–14.
Grossman MD, Miller D, Scaff DW, Arcona S. When is an elder old? Effect of preexisting conditions on mortality in geriatric trauma. J Trau Inj Infect Crit Care. 2002;52(2):242–6.
Morris JA Jr, MacKenzie EJ, Edelstein SL. The effect of preexisting conditions on mortality in trauma patients. J Am Med Assoc. 1990;263(14):1942–6.
Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, De Boer IH, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–21.
Courtney-Brooks M, Tellawi AR, Scalici J, Duska LR, Jazaeri AA, Modesitt SC, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol. 2012;126(1):20–4.
Mack M. Frailty and aortic valve disease. J Thorac Cardiovasc Surg. 2013;145(3 Suppl.):S7–10.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci. 2001;56(3):M146–56.
Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Sayer AA, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23(7):1839–48.
Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–8. doi:10.1016/j.jamcollsurg.2010.03.039.
Lee EA. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53:912–7.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (RED Cap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Brown NA, Zenilman ME. The impact of frailty in the elderly on the outcome of surgery in the aged. Adv Surg. 2010;44(1):229–49.
Yeguiayan JM, Yap A, Freysz M, Garrigue D, Jacquot C, Martin C et al. Impact of whole-body computed tomography on mortality and surgical management of severe blunt trauma. Crit Care. 2012;16(3):R101. doi:10.1186/cc11375.
Lee JSJ, He K, Harbaugh CM, Schaubel DE, Sonnenday CJ, Wang SC, et al. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53(4):912–7.
Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus. 2013 (abstract).
Peng PD, Van Vledder MG, Tsai S, De Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB. 2011;13(7):439–46.
De Fanis U, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008;56(5):904–8. doi:10.1111/j.1532-5415.2008.01673.x.
Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46(3):319–24. doi:10.1016/j.cyto.2009.02.015.
Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol Series A Biol Sci Med Sci. 2009;64(4):499–502. doi:10.1093/gerona/gln047.
Conflict of interest
Laura Ebbeling, Daniel J Grabo, Michael Shashaty, Ruchicka Dua, Seema S. Sonnad, Carrie A. Sims, Jose L. Pascual, C. William Schwab, and Daniel N. Holena declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
# | Complication | Description |
|---|---|---|
Pulmonary | ||
20 | Acute respiratory distress syndrome (ARDS)* *(maps to NTD B definition) | Adult (Acute) respiratory distress syndrome: ARDS occurs in conjunction with catastrophic medical conditions, such as pneumonia, shock, sepsis (or severe infection throughout the body, sometimes also referred to as systemic infection, and may include or also be called a blood or blood-borne infection), and trauma. It is a form of sudden and often severe lung failure characterized by PaO2/FiO2 ≤200, decreased compliance, and diffuse bilateral pulmonary infiltrates without associated clinical evidence of CHF. The process must persist beyond 36 hours and require mechanical ventilation. |
21 | Acute respiratory failure | Need for prolonged ventilatory support after a period of normal non-assisted breathing (minimum of 48 hrs) or reintubation. a.Planned—do not report (i.e., taken to OR) b.Unplanned––report |
22 | Aspiration/aspiration pneumonia | Documented inhalation of gastric contents or other materials followed by clinical and new radiological findings of pneumonitis which requires treatment within 48 h. |
24 | Fat embolus syndrome | Documented diagnosis by an attending physician in a patient w/pelvic or extremity fractures and a decreased PO2. One of the following must also be present: change in mental status; petechial signs; tachypnea; fat in urine; decreased platelets. |
26 | Pneumonia* *(maps to NTDB definition) | Patients with evidence of pneumonia that develops during the hospitalization. Patients with pneumonia must meet at least one of the following two criteria: Criterion 1. Rales or dullness to percussion on physical examination of chest AND any of the following: a. New onset of purulent sputum or change in character of sputum b. Organism isolated from blood culture c. Isolation of pathogen from specimen obtained by transtracheal aspirate, bronchial brushing, or biopsy Criterion 2. Chest radiographic examination shows new or progressive infiltrate, consolidation, cavitation, or pleural effusion AND any of the following: a. New onset of purulent sputum or change in character of sputum b. Organism isolated from the blood c. Isolation of pathogen from specimen obtained by transtracheal aspirate, bronchial brushing, or biopsy d. Isolation of virus or detection of viral antigen in respiratory secretions e. Diagnostic single antibody titer (IgM) or fourfold increase in paired serum samples (IgG) for pathogen f. Histopathologic evidence of pneumonia |
27 | Pneumothorax | Presence of intrapleural air not present on admission radiograph, resulting from treatment or intervention. |
28 | Pulmonary embolus* *(maps to NTDB definition) | Defined as a lodging of a blood clot in a pulmonary artery with subsequent obstruction of blood supply to the lung parenchyma. The blood clots usually originate from the deep veins or the pelvic venous system. Consider the condition present if the patient has a VQ scan interpreted as high probability of pulmonary embolism or a positive pulmonary arteriogram or positive CT angiogram. |
48 | Cardiopulmonary arrest (unexpected, not resulting in death) | Documented by a physician. |
Cardiovascular | ||
30 | Acute arterial occlusion (not present on admission) | Caused by embolism or thrombosis, documented by arteriography, duplex scanning, pulse volume recording, segmental pressures, clinical exam, or autopsy. |
34 | Major dysrhythmia (not resulting in death) | Dysrhythmia requiring drugs or defibrillation (e.g., supraventricular tachycardia, rapid atrial fibrillation, sustained ventricular tachycardia, bradycardia requiring pacing). |
32 | Extremity compartment syndrome (not present on admission)* *(maps to NTDB definition) | Defined as a condition in which there is swelling and an increase in pressure within a limited space (a fascial compartment) that presses on and compromises blood vessels, nerves, and/or tendons that run through that compartment. Compartment syndromes usually involve the leg but can also occur in the forearm, arm, thigh, and shoulder. |
33 | Deep vein thrombosis (DVT)* *(maps to NTDB definition) | The formation, development, or existence of a blood clot or thrombus within the vascular system, which may be coupled with inflammation. This diagnosis may be confirmed by a venogram, ultrasound, or CT. The patient must be treated with anticoagulation therapy and/or placement of a vena cava filter or clipping of the vena cava. |
35 | Myocardial infarction (MI)* *(maps to NTDB definition) | A new acute myocardial infarction occurring during hospitalization (within 30 days of injury). |
Hematologic/coagulopathy | ||
40 | Blood transfusion reaction | Transfusion reaction as documented by the institution’s reaction protocol |
41 | Coagulopathy (excluding anticoagulation therapy, coumadin therapy, or underlying hematologic disorders, e.g., hemophilia) | Uncontrolled diffuse bleeding in the presence of coagulation abnormalities, e.g., increased prothrombin time, increased partial thromboplastin time, decreased platelet count, or disseminated intravascular coagulation (DIC) requiring treatment, i.e., transfusion components such as platelets, clotting factors, FFP. |
Renal | ||
50 | Acute renal failure* *(maps to NTDB definition) | A patient who did not require dialysis prior to injury, and who has worsening renal dysfunction after an injury requiring hemodialysis, ultrafiltration, or peritoneal dialysis. If the patient refuses treatment (e.g., dialysis), the condition is still considered present. |
Hepatic | ||
63 | Liver failure | Documented by a physician. Increased serum ammonium or decreased synthetic or metabolic function (e.g., PT, TTP, or fibrinogen). |
Infection/sepsis | ||
70 | Empyema | Infection documented by purulent material or positive culture from the pleural space, requiring thoracostomy tube drainage. |
76 | Sepsis | Documented by a physician with at least two or more of the following conditions (which occur at the same time): core temperature of >38 or ≤36°C; white blood cell count >12,000 or <4,000 or >10% immature bands; positive blood cultures (excluding contaminants); clinically obvious source of infection; heart rate >90 beats/min or respiratory rate >20 breaths/min. |
77 | Septicemia | (+) blood cx, excluding isolates thought to be contaminates. |
78 | Acute sinusitis | Opacification on XRAY or CT with fever and/or positive purulent drainage requiring treatment. |
79 | Soft tissue infection | Documentation by a physician of cellulitis, gas gangrene, necrotizing fasciitis, or streptococcal myositis requiring treatment. |
97 | Urinary tract infection (UTI) (not present on admission) (CDC guidelines used as reference) | Clean voided or other catheter urine specimen with ≥100,000 organisms/ml on C/S. |
99 | Wound infection | (Traumatic or incisional) drainage of purulent material from wound; active treatment of wound, or administration of antibiotics for wound. *An abdominal abscess would not be considered a wound infection and is not applicable as an occurrence. |
Airway management | ||
80 | Esophageal intubation | Endotracheal tube in esophagus and not immediately repositioned. Esophageal location determined by physical exam, X-ray, capnography, or endoscopy. |
69 | Unrecognized main stem bronchus intubation | Any endotracheal intubation procedure resulting in definitive placement of the tube in either the right or left main stem bronchus. a.Recognized and treated immediately—not reportable b.Unrecognized on two successive chest xrays––reportable |
Gastrointestinal | ||
83 | GI bleeding | Blood loss from anywhere in GI tract, grossly positive nasogastric (NG) aspirate, or grossly positive stool which requires treatment. |
86 | Small bowel obstruction (SBO) (excluding ileus) | Radiographic evidence of dilated loop of bowel with multiple air-fluid levels and confirmed by a surgeon; requiring treatment (surgery or NG tube). |
Neurological | ||
64 | CNS infection | CSF aspirate with positive culture and increased white blood cell count. |
66 | Progression of original neurologic insult | Documentation by a physician of deterioration or additional loss of function from that noted on ED arrival, i.e., paralysis, paresis, or other neurologic sequelae. |
96 | Seizures | Two or more seizures after a head injury which are witnessed or attested to by hospital personnel (e.g., focal, grand mal, conscious or unconscious (one seizure after a head injury is not a complication; 10–15% are expected). |
Procedure-related | ||
91 | Organ, nerve, vessel injury | Perforation or injury resulting from treatment or intervention. |
Decubitus | ||
65 | Dehiscence/evisceration | Breakdown of fascial closure confirmed by discharge of peritoneal fluid, evisceration, or palpable fascial defect. *THIS OCCURRENCE PERTAINS TO THE ABDOMINAL AREA ONLY* |
94 | Decubitus* *(maps to NTDB definition) | Defined as a “pressure sore” resulting from pressure exerted on the skin, soft tissue, muscle, or bone by the weight of an individual against a surface beneath. Individuals unable to avoid long periods of uninterrupted pressure over bony prominences are at increased risk for the development of necrosis and ulceration. |
Hypothermia | ||
46 | Hypothermia | (Non-therapeutic) rectal or core temperature ≤34 °C or 93.2 °F. If the patient presents to the hospital with hypothermia, the hypothermia is considered a diagnosis. If the hypothermia presents during the hospital stay and is unexpected, the hypothermia is considered an occurrence. |
47 | Postoperative hemorrhage | Requiring operative intervention—procedures done in angio to control hemorrhage should be considered operative interventions and the hemorrhage should be included as an occurrence. |
49 | Adverse drug reaction | Documented by a physician, plus one of the following: adversely affects patient care; increases length of stay; increases morbidity and mortality. |
Rights and permissions
About this article
Cite this article
Ebbeling, L., Grabo, D.J., Shashaty, M. et al. Psoas:lumbar vertebra index: central sarcopenia independently predicts morbidity in elderly trauma patients. Eur J Trauma Emerg Surg 40, 57–65 (2014). https://doi.org/10.1007/s00068-013-0313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-013-0313-3