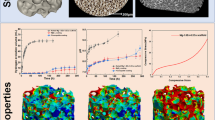
Osteoblast phenotypic expression in monolayer culture depends on surface microtopography. Here we tested the hypothesis that mineralized bone nodule formation in response to osteotropic agents such as bone morphogenetic protein-2 (BMP-2) and dexamethasone is also influenced by surface microtopography. Fetal rat calvarial (FRC) cells were cultured on Ti implant materials (PT [pretreated], Ra = 0.6 mm; SLA [course grit blasted and acid etched], Ra = 4.0 mm; TPS [Ti plasma sprayed], Ra = 5.2 mm) in the presence of either BMP-2 (20 ng/ml) or 10?8 M dexamethasone (Dex). At 14 days post-confluence, a homogenous layer of cells covered the surfaces, and stacks of cells that appeared to be nodules emerging from the culture surface were present in some areas on all three Ti surfaces. Cell proliferation decreased while alkaline phosphatase specific activity (ALPase) and nodule number generally increased with increasing surface roughness in both control and treated cultures. There was no difference in cell number between the control and Dex-treated cultures for a particular surface, but BMP-2 significantly reduced cell number compared with control or Dex-treated cultures. Treatment with Dex or BMP-2 further increased ALPase on all surfaces except for PT cultures with Dex. Dex had no effect on nodule area in cultures grown on PT or SLA disks, yet increased nodule number by more than 100% in cultures on PT disks. Though the effect of BMP-2 on nodule number was the same as Dex, BMP-2 increased nodule area on all surfaces except TPS, where area was decreased. Ca and P content of the cell layers in control cultures did not vary with surface roughness. However, cultures treated with Dex had increased Ca content on all surfaces, but the greatest increase was seen on SLA and TPS. BMP-2 increased Ca content in cultures on all surfaces, with the greatest increase on the PT surface. BMP-2 treatment increased P content on all surfaces, whereas Dex only increased P on rough surfaces. Of all cultures examined, the Ca/P weight ratio was 2:1 only on rough surfaces with BMP-2, indicating the presence of bone-like apatite. This was further validated by Fourier transform infrared (FTIR) imaging showing a close association between mineral and matrix on TPS and SLA surfaces with BMP-2-treated cells, and individual spectra indicated the presence of an apatitic mineral phase comparable to bone. In contrast, mineral on the smooth surface of BMP-2-treated cultures and on all surfaces where cultures were treated with Dex was not associated with the matrix and the spectra, not typical of bone apatite, implying dystrophic mineralization. This demonstrates that interactions between growth factor or hormone and surface microtopography can modulate bone cell differentiation and mineralization.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boyan, B., Bonewald, L., Paschalis, E. et al. Osteoblast-Mediated Mineral Deposition in Culture is Dependent on Surface Microtopography . Calcif Tissue Int 71, 519–529 (2002). https://doi.org/10.1007/s00223-001-1114-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00223-001-1114-y