Abstract
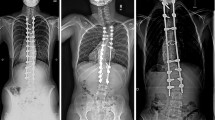
The objective of this study is to compare the clinical and radiographic results of ultraporous β-tricalcium phosphate (β-TCP) versus autogenous iliac crest bone graft (ICBG), through prospective randomized pilot study (EBM-Level 1), as graft extenders in scoliosis surgery. In the posterior correction of scoliosis, local bone resected as part of the procedure is used as the base bone graft material. Supplemental grafting from the iliac crest is considered the gold-standard in posterior spinal fusion. However, autograft is not available in unlimited quantities, and bone harvesting is a source of significant morbidity. Ultraporous β-TCP might be a substitute for ICBG in these patients and thus eliminate donor site morbidity. A total of 40 patients with adolescent idiopathic scoliosis (AIS) were randomized into two treatment groups and underwent corrective posterior instrumentation. In 20 patients, ICBG harvesting was performed whereas the other half received β-TCP (VITOSS®) to augment the local bone graft. If thoracoplasty was performed, the resected rib bone was added in both groups. Patients were observed clinically and radiographically for a minimum of 20 months postoperatively, with a mean follow-up of 4 years. Overall pain and pain specific to the back and donor site were assessed using a visual analog scale (VAS). As a result, both groups were comparable with respect to the age at the time of surgery, gender ratio, preoperative deformity, and hence length of instrumentation. There was no significant difference in blood loss and operative time. In nine patients of the β-TCP group and eight patients of the ICBG group, thoracoplasty was performed resulting in a rib graft of on average 7.9 g in both groups. Average curve correction was 61.7% in the β-TCP group and 61.2% in the ICBG group at hospital discharge (P = 0.313) and 57.2 and 54.3%, respectively, at follow-up (P = 0.109). Loss of curve correction amounted on average 2.6° in the β-TCP group and 4.2° in the comparison group (P = 0.033). In the ICBG group, four patients still reported donor site pain of on average 2/10 on the VAS at last follow-up. One patient in the β-TCP group was diagnosed with a pseudarthrosis at the caudal end of the instrumentation. Revision surgery demonstrated solid bone formation directly above the pseudarthrosis with no histological evidence of β-TCP in the biopsy taken. In conclusion, the use of β-TCP instead of ICBG as extenders of local bone graft yielded equivalent results in the posterior correction of AIS. The promising early results of this pilot study support that β-TCP appears to be an effective bone substitute in scoliosis surgery avoiding harvesting of pelvic bone and the associated morbidity.





Similar content being viewed by others
References
An HS, Lynch K, Toth J (1995) Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord 8:131–135. doi:10.1097/00002517-199504000-00007
Arrington ED, Smith WJ, Chambers HG et al (1996) Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 329:300–309. doi:10.1097/00003086-199608000-00037
Aurori BF, Weierman RJ, Lowell HA et al (1985) Pseudarthrosis after spinal fusion for scoliosis. A comparison of autogeneic and allogeneic bone grafts. Clin Orthop Relat Res 199:153–158
Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 20:1055–1060. doi:10.1097/00007632-199505000-00012
Betz RR (2002) Limitations of autograft and allograft: new synthetic solutions. Orthopedics 25:s561–s570
Betz RR, Petrizzo AM, Kerner PJ et al (2006) Allograft versus no graft with a posterior multisegmented hook system for the treatment of idiopathic scoliosis. Spine 31:121–127. doi:10.1097/01.brs.0000194771.49774.77
Bucholz RW (2002) Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 395:44–52. doi:10.1097/00003086-200202000-00006
Cavagna R, Daculsi G, Bouler JM (1999) Macroporous calcium phosphate ceramic: a prospective study of 106 cases in lumbar spinal fusion. J Long Term Eff Med Implants 9:403–412
Chen WJ, Tsai TT, Chen LH et al (2005) The fusion rate of calcium sulfate with local autograft bone compared with autologous iliac bone graft for instrumented short-segment spinal fusion. Spine 30:2293–2297. doi:10.1097/01.brs.0000182087.35335.05
Cobb J (1948) Outline for the study of scoliosis. In: Instructional course letters, vol 5. American Academy of Orthopaedic Surgeons, Ann Arbor
Delecrin J, Takahashi S, Gouin F et al (2000) A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine 25:563–569. doi:10.1097/00007632-200003010-00006
Dodd CA, Fergusson CM, Freedman L et al (1988) Allograft versus autograft bone in scoliosis surgery. J Bone Joint Surg Br 70:431–434
Eastlund T (2006) Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Bank 7:147–166. doi:10.1007/s10561-006-0003-z
Ehrler DM, Vaccaro AR (2000) The use of allograft bone in lumbar spine surgery. Clin Orthop Relat Res 371:38–45. doi:10.1097/00003086-200002000-00005
Epstein NE (2006) A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech 19:424–429. doi:10.1097/00024720-200608000-00009
Erbe EM, Marx JG, Clineff TD et al (2001) Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur Spine J 10(Suppl 2):S141–S146. doi:10.1007/s005860100288
Fabry G (1991) Allograft versus autograft bone in idiopathic scoliosis surgery: a multivariate statistical analysis. J Pediatr Orthop 11:465–468
Fernyhough JC, Schimandle JJ, Weigel MC et al (1992) Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine 17:1474–1480
Fleming JE Jr, Cornell CN, Muschler GF (2000) Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am 31:357–374. doi:10.1016/S0030-5898(05)70156-5
Gibson S, McLeod I, Wardlaw D et al (2002) Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: a randomized control trial. Spine 27:1599–1603. doi:10.1097/00007632-200208010-00002
Goulet JA, Senunas LE, DeSilva GL et al (1997) Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res 339:76–81. doi:10.1097/00003086-199706000-00011
Gunzburg R, Szpalski M (2002) Use of a novel beta-tricalcium phosphate-based bone void filler as a graft extender in spinal fusion surgeries. Orthopedics 25:s591–s595
Henman P, Finlayson D (2000) Ordering allograft by weight: suggestions for the efficient use of frozen bone-graft for impaction grafting. J Arthroplasty 15:368–371. doi:10.1016/S0883-5403(00)90785-X
Ibrahim T, Stafford H, Esler CN et al (2004) Cadaveric allograft microbiology. Int Orthop 28:315–318. doi:10.1007/s00264-004-0579-5
Jorgenson SS, Lowe TG, France J et al (1994) A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine 19:2048–2053
Journeaux SF, Johnson N, Bryce SL et al (1999) Bacterial contamination rates during bone allograft retrieval. J Arthroplasty 14:677–681. doi:10.1016/S0883-5403(99)90222-X
King HA, Moe JH, Bradford DS et al (1983) The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am 65:1302–1313
Kwon B, Jenis LG (2005) Carrier materials for spinal fusion. Spine J 5:224–230. doi:10.1016/j.spinee.2005.02.007
Le Huec JC, Lesprit E, Delavigne C et al (1997) Tri-calcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthop Belg 63:202–211
Liljenqvist U, Lepsien U, Hackenberg L et al (2002) Comparative analysis of pedicle screw and hook instrumentation in posterior correction and fusion of idiopathic thoracic scoliosis. Eur Spine J 11:336–343. doi:10.1007/s00586-002-0415-9
Linovitz RJ, Peppers TA (2002) Use of an advanced formulation of beta-tricalcium phosphate as a bone extender in interbody lumbar fusion. Orthopedics 25:s585–s589
McLain RF, Fleming JE, Boehm CA et al (2005) Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am 87:2655–2661. doi:10.2106/JBJS.E.00230
Meadows GR (2002) Adjunctive use of ultraporous beta-tricalcium phosphate bone void filler in spinal arthrodesis. Orthopedics 25:s579–s584
Muschik M, Ludwig R, Halbhubner S et al (2001) Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J 10(Suppl 2):S178–S184. doi:10.1007/s005860100271
Muschler GF, Matsukura Y, Nitto H et al (2005) Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res 432:242–251. doi:10.1097/01.blo.0000149812.32857.8b
Muschler GF, Midura RJ (2002) Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res 395:66–80. doi:10.1097/00003086-200202000-00008
Price CT, Connolly JF, Carantzas AC et al (2003) Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine 28:793–798. doi:10.1097/00007632-200304150-00012
Ransford AO, Morley T, Edgar MA et al (1998) Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br 80:13–18. doi:10.1302/0301-620X.80B1.7276
Robertson PA, Wray AC (2001) Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine 26:1473–1476. doi:10.1097/00007632-200107010-00018
Sandhu HS, Grewal HS, Parvataneni H (1999) Bone grafting for spinal fusion. Orthop Clin North Am 30:685–698. doi:10.1016/S0030-5898(05)70120-6
Sasso RC, LeHuec JC, Shaffrey C (2005) Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech 18(Suppl):S77–S81. doi:10.1097/01.bsd.0000112045.36255.83
Sengupta DK, Truumees E, Patel CK et al (2006) Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine 31:985–991. doi:10.1097/01.brs.0000215048.51237.3c
Skaggs DL, Samuelson MA, Hale JM et al (2000) Complications of posterior iliac crest bone grafting in spine surgery in children. Spine 25:2400–2402. doi:10.1097/00007632-200009150-00021
Summers BN, Eisenstein SM (1989) Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br 71:677–680
Violas P, Chapuis M, Bracq H (2004) Local autograft bone in the surgical management of adolescent idiopathic scoliosis. Spine 29:189–192. doi:10.1097/01.BRS.0000105536.65164.B1
Wippermann BW, Schratt HE, Steeg S et al (1997) Complications of spongiosa harvesting of the ilial crest. A retrospective analysis of 1,191 cases. Chirurg 68:1286–1291. doi:10.1007/s001040050361
Woolf SK, Gross RH (2001) Perceptions of allograft safety and efficacy among spinal deformity surgeons. J Pediatr Orthop 21:767–771. doi:10.1097/00004694-200111000-00013
Acknowledgments
The authors thank Randal R. Betz, MD, Shriners Hospitals for Children, Philadelphia, for his valuable help and contribution in reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lerner, T., Bullmann, V., Schulte, T.L. et al. A level-1 pilot study to evaluate of ultraporous β-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J 18, 170–179 (2009). https://doi.org/10.1007/s00586-008-0844-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-008-0844-1