Abstract
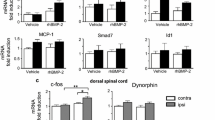
A novel rat model was used to investigate the effect of nitric oxide synthase inhibition in posterior spinal fusion augmented with recombinant human bone morphogenetic protein-2. Nitric oxide (NO) has important physiological functions including the modulation of fracture healing. Recombinant human BMP-2 (rhBMP-2) enhances spinal fusion. It is not known whether nitric oxide has a role in rhBMP-2 enhanced spinal fusion and remodeling. A novel rat intertransverse fusion model was created using a defined volume of bone graft along with a collagen sponge carrier, which was compacted and delivered using a custom jig. The control groups consisted of a sham group (S, n = 20), an autograft + carrier group (A, n = 28) and a group consisting of 43 μg of rhBMP-2 mixed with autograft + carrier (AB, n = 28). Two experimental groups received a nitric oxide synthase (NOS) inhibitor, N G-nitro l-arginine methyl ester, in a dose of 1 mg/ml ad lib in the drinking water (AL, n = 28) and one of these experimental groups had rhBMP-2 added to the graft mixture at the time of surgery (ALB, n = 28). Rats were killed at 22 and 44 days, spinal columns subjected to radiology, biomechanics and histology. On a radiographic score (0–4) indicating progressive maturation of bone fusion mass, no difference was found between the A and AL groups, however, there was a significant enhancement of fusion when rhBMP-2 was added when compared to the A group (P < 0.001). However, on day 44, the ALB group showed significantly less fusion progression when compared to the AB group (P < 0.01). There was a 25% (P < 0.05) more fusion-mass-area in day 44 of ALB group when compared to day 44 of the AB group indicating that NOS inhibition delayed the remodeling of the fusion mass. Biomechanically, the rhBMP-2 groups were stiffer at all time points compared to the NOS inhibited groups. Decalcified histology demonstrated that there was a delay in graft incorporation whenever NOS was inhibited (AL and ALB groups) as assessed by a 5 point histological maturation score. In a novel model of rat intertransverse process fusion, nitric oxide synthase modulates rhBMP-2 induced corticocancellous autograft incorporation.







Similar content being viewed by others
References
Diwan AD, Wang MX, Jang D et al (2000) Nitric oxide modulates fracture healing. J Bone Miner Res 15(2):342–351
Zhu W, Diwan AD, Lin JH et al (2001) Nitric oxide synthase isoforms during fracture healing. J Bone Miner Res 16(3):535–540
Zhu W, Murrell GA, Lin JH et al (2002) Localization of nitric oxide synthases during fracture healing. J Bone Miner Res 17(8):1470–1477
Tsuji K, Bandyopadhyay A, Harfe BD et al (2006) BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38(12):1424–1429
Murnaghan M, McIlmurray L, Mushipe M et al (2005) Time for treating bone fracture using rhBMP-2: a randomised placebo controlled mouse fracture trial. J Orthop Res 23(3):625–631
Sumner DR, Turner TM, Urban RM et al (2004) Locally delivered rhBMP-2 enhances bone ingrowth and gap healing in a canine model. J Orthop Res 22(1):58–65
Haid RW Jr, Branch CL Jr, Alexander JT et al (2004) Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 4(5):527–538
Burkus JK, Sandhu HS, Gornet MF et al (2005) Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am 87(6):1205–1212
Jone AL, Buchholtz RW, Bosse MJ et al (2006) Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am 88(7):1431–1441
Singh K, Smucker JD, Boden SD et al (2006) Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion: a prospective CT-scan analysis at one and two years. J Spinal Disord Tech 19(6):416–423
Morisue H, Matsimoto M, Chiba K et al (2006) A novel hydroxyapatite fiber mesh as a carrier for recombinant human bone morphogenetic protein-2 enhances bone union in rat posterolateral fusion model. Spine 31(11):1194–1200
Carlisle E, Fischgrund JS (2005) Bone morphogenetic proteins for spinal fusion. Spine J 5(6 Suppl):240S–249S
Grauer JN, Bomback DA, Lugo R et al (2004) Posterolateral lumbar fusions in athymic rats: characterization of a model. Spine J 4(3):281–286
Zhu W, Rawlins BA, Boachie-Adjei O et al (2004) Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res 19(12):2021–2032
Salamon ML, Althausen PL, Gupta MC et al (2003) The effects of BMP-7 in a rat posterolateral intertransverse process fusion model. J Spinal Disord Tech 16(1):90–95
Bomback DA, Grauer JN, Lugo R et al (2004) Comparison of posterolateral lumbar fusion rates of Grafton Putty and OP-1 Putty in an athymic rat model. Spine 29(15):1612–1617
Boden SD, Titus L, Hair G et al (1998) Lumbar spine fusion by local gene therapy with cDNA encoding a novel osteoinductive protein (LMP-1). Spine 23:2486–2492
Wang JC, Davies M, Kanim LEA, Ukatu CJ, Dawson EG, Lieberman JR (2000) Prospective comparison of commercially available demineralized bone matrix for spine fusion. Paper presented at North American Spine Society Meeting; October 25–28, 2000, New Orleans, LA, USA
Boden SD, Schimandle JH, Hutton WC et al (1995) An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine 20(4):412–420
Boden SD (2002) Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine 27(16 Suppl 1):S26–S31
Jung JY, Lin AC, Ramos LM et al (2003) Nitric oxide synthase I mediates osteoclast activity in vitro and in vivo. J Cell Biochem 89(3):613–621
Acknowledgments
The authors would like to acknowledge Deirdre Campbell D.Eng, Russel Nord, BS, Micheal Peterkin, BS and Steven B. Doty, PhD for their invaluable assistance in the completion of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diwan, A.D., Khan, S.N., Cammisa, F.P. et al. Nitric oxide modulates recombinant human bone morphogenetic protein-2-induced corticocancellous autograft incorporation: a study in rat intertransverse fusion. Eur Spine J 19, 931–939 (2010). https://doi.org/10.1007/s00586-009-1263-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-009-1263-7