Abstract
Purpose
This study aims to investigate the differential expression proteins profile of spinal cord tissues after acute spinal cord injury (ASCI), provide preliminary results for further study and explore the secondary injury mechanisms underlying ASCI.
Methods
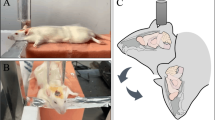
Using Allen’s frame to establish ASCI model of Sprague–Dawley rats, then a stable isotope-labelled strategy using isobaric tags for relative and absolute quantitation (iTRAQ) coupled with two-dimensional (2D) liquid chromatography tandem mass spectrometry (2D LC–MS/MS) was performed to separate and identify differentially expressed proteins.
Results
A total of 220 differentially expressed proteins were identified in the spinal cord tissues of H-8 group (acute spinal cord injury after 8 h) compared with H-0 group (acute spinal cord injury after 0 h); Up to 116 proteins were up-regulated, whereas 104 proteins were down-regulated in the spinal cord tissues. Three of the differentially expressed Heat shock proteins (HSPs) namely, Hsp90ab1, Hspa4 and Hspe1 were down-regulated.
Conclusion
The differentially expressed proteins of spinal cord tissues after ASCI will provide scientific foundation for further study to explore the secondary injury mechanism of ASCI.



Similar content being viewed by others
References
Hagg T, Oudega M (2006) Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma 23:264–280
McBride CB, McPhail LT, Vanderluit JL et al (2003) Caspase inhibition attenuates transection-induced oligodendrocyte apoptosis in the developing chick spinal cord. Mol Cell Neurosci 23:383–397
Whalley K, O’Neill P, Ferretti P (2006) Changes in response to spinal cord injury with development: vascularization, haemorrhage and apoptosis. Neuroscience 137:821–832
Carmel JB, Galante A, Soteropoulos P et al (2001) Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol Genomics 7:201–213
Morange M (2005) What history tells us II. The discovery of chaperone function. J Biosci 30:461–464
Campisi J, Fleshner M (2003) Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol 94:43–52
Robinson MB, Tidwell JL, Gould T et al (2005) Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci 25:9735–9745
Chen Y, Voegeli TS, Liu PP et al (2007) Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm Allergy Drug Targets 6:91–100
Sangala JR, Frankla MR, Paul P (2008) The role of heat shock proteins in spinal cord injury. Neurosurg Focus 25(5):E4
Yan W, Chen SS (2005) Mass spectrometry-based quantitative proteomic profiling. Brief Funct Genomic Proteomic 4:27–38
Ross PL, Huang YN, Marchese JN et al (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using aminereactive isobaric tagging reagents. Mol Cell Proteomics 3:1154–1169
Wu WW, Wang G, Baek SJ et al (2006) Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel-or LC-MALDI TOF/TOF. J Proteome Res 5:651–658
Shadforth IP, Dunkley TP, Lilley KS et al (2005) i-Tracker: for quantitative proteomics using iTRAQ[J]. BMC Genomics 6:145
Ernoult E, Gamelin E, Guette C (2008) Improved proteome coverage by using iTRAQ labelling and peptide OFFGEL fractionation. Proteome Sci 6:27
Molloy MP (2000) Two-dimensional electrophoresis of membrane proteins using immobilized pH gradients. Anal Biochem 280:1–10
Rabilloud T (2009) Membrane proteins and proteomics: love is possible, but so difficult. Electrophoresis 30 Supp l:S174–S180
Choo AM, Liu J, Liu Z et al (2009) Modeling spinal cord contusion, dislocation, and distraction: characterization of vertebral clamps, injury severities, and node of Ranvier deformations. J Neurosci Methods 181:6–17
Byrnes KR, Stoica B, Riccio A et al (2009) Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents. Ann Neurol 66(1):63–74
Ross PL, Huang YN, Marchese JN et al (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3:1154–1169
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Awad H, Suntres Z, Heijmans J et al (2008) Intracellular and extracellular expression of the major inducible 70 kDa heat shock protein in experi-mental ischemia-reperfusion injury of the spinal cord[J]. Exp Neurol 212:275–284
Kennaway CK, Benesch JL, Gohlke U et al (2005) Dodecameric structure of the small heat shock protein Acr1 from Mycobacterium tuberculosis. J Biol Chem 280:33419–33425
Kumar R, Pavithra SR, Tatu U (2007) Three-dimensional structure of heat shock protein 90 from Plasmodium falciparum: molecular modelling approach to rational drug design against malaria. J Biosci 32:531–536
Guzman-Lenis MS, Vallejo C, Navarro X et al (2008) Analysis of FK506-mediated protection in an organotypic model of spinal cord damage: heat shock protein 70 levels are modulated in microglial cells. Neuroscience 155:104–113
Tator CH, Fehlings MG (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75:15–26
Richter K, Buchner J (2001) Hsp90: chaperoning signal transduction. J Cell Physiol 188:281–290
Conroy SE, Latchman DS (1996) Do heat shock proteins have a role in breast cancer? Br J Cancer 74:717–721
Chopp M, Chen H, Ho KL et al (1989) Transient hyperthermia protects against subsequent forebrain ischemic cell damage in the rat. Neurology 39:1396–1398
Cid C et al (2004) Antibodies reactive to heat shock protein 90 induce oligodendrocyte precursor cell death in culture. Implications for demyelination in multiple sclerosis. FASEB J 18:409–411
Cid C, Alvarez-Cermeno JC et al (2005) Anti-heat shock protein 90β antibodies decrease preoligodendrocyte population in perinatal and adult cell cultures. Implications for remyelination in multiple sclerosis. J Neurochem 95:349–360
Bergamaschini L, Cicardi M (2003) Recent advances in the use of C1 inhibitor as a therapeutic agent. Mol Immunol 40:155–158
Brown IR, Rush S, Ivy GO (1989) Induction of a heat shock gene at the site of tissue injury in the rat brain. Neuron 2:1559–1564
Lindquist S (1986) The heat-shock response. Ann Rev Biochem 55:1151–1191
Popovich PG, Wei P, Stokes BT (1997) Cellular inflammatory response after spinal cord injury in Sprague–Dawley and Lewis rats. J Comp Neurol 377:443–464
Beckmann RP, Mizzen LE, Welch WJ (1990) Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 248:850–854
Chopp M, Chen H, Ho KL et al (1989) Transient hyperthermia protects against subsequent forebrain ischemic cell damage in the rat. Neurology 39:1396–1398
Kawagoe J, Abe K, Sato S et al (1992) Distributions of heat shock protein-70 mRNAs and heat shock cognate protein-70 mRNAs after transient global ischemia in gerbil brain. J Cereb Blood Flow Metab 2:794–801
Plumier JC, Krueger AM, Currie RW et al (1997) Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones 2:162–167
Hartman DJ, Hoogenraad NJ, Condron R et al (1992) Identification of a mammalian 10-kDa heat shock protein, a mitochondrial chaperonin 10 homologue essential for assisted folding of trimeric ornithine transcarbamoylase in vitro. Proc Natl Acad Sci USA 89:3394–3398
Rospert S, Glick BS, Jeno P et al (1993) Identification and functional analysis of chaperonin 10, the groES homolog from yeast mitochondria. Proc Natl Acad Sci USA 90:10967–10971
Czarnecka AM, Campanella C, Zummo G et al (2006) Heat shock protein 10 and signal transduction: a “capsula eburnean” of carcinogenesis? Cell Stress Chaperones 11:287–294
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Richardson A, Landry SJ, Georgopoulos C (1998) The ins and outs of a molecular chaperone machine. Trends Biochem Sci 23:138–143
Barazi HO, Zhou L, Templeton NS et al (2002) Identification of heat shock protein 60 as a molecular mediator of alpha 3 beta 1 integrin activation. Cancer Res 62:1541–1548
Shin BK, Wang H, Yim AM et al (2003) Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem 278:7607–7616
Kloetzel PM, Ossendorp F (2004) Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr Opin Immunol 16(1):76–81
Kerlero de Rosbo N, Milo R, Lees MB et al (1993) Reactivity to myelin antigens in multiple sclerosis. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest 92(6):2602–2608
Sun J, Link H, Olsson T et al (1991) T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. J Immunol 146(5):1490–1495
Zhang Y, Burger D, Saruhan G et al (1993) The T-lymphocyte response against myelinassociated glycoprotein and myelin basic protein in patients with multiple sclerosis. Neurology 43(2):403–407
Jain MR, Li Q, Liu T et al (2012) Proteomic identification of immunoproteasome accumulation in formalin-fixed rodent spinal cords with experimental autoimmune encephalomyelitis. J Proteome Res 11(3):1791–1803
Tsuchida T, Parker KC, Turner RV et al (1994) Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc Natl Acad Sci USA 91(23):10859–10863
Ito Y, Sugimoto Y, Tomioka M et al (2009) Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine 34(20):2121–2124
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Zhou and L. Xu contributed equally to this paper and are also co-first authors for this article.
Rights and permissions
About this article
Cite this article
Zhou, Y., Xu, L., Song, X. et al. The potential role of heat shock proteins in acute spinal cord injury. Eur Spine J 23, 1480–1490 (2014). https://doi.org/10.1007/s00586-014-3214-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3214-1