INTRODUCTION
Vertebral osteomyelitis (VO) is defined as infection of the intervertebral disc and adjacent vertebrae. VO accounts for 2–4% of bone and joint infections [Reference Jevtic1–Reference Tali3]. The disease has a male predominance with a male:female ratio of 1·5 [Reference Jevtic1, Reference Tali3]. It can affect persons of all ages, although it occurs most frequently in the fifth or sixth decade [Reference Jevtic1, Reference Tali3, Reference Smith and Blaser4]. The reported mortality in published studies ranges from 2% to 30% [Reference Tali3, Reference Smith and Blaser4]. The most common causative microorganisms are Staphylococcus aureus, in 40–60% of cases [Reference Khan, Vaccaro and Zlotolow2, Reference Tali3, Reference Hopkinson, Stevenson and Benjamin5], Gram-negative bacilli including enterobacteria (20–30%) and streptococci [Reference Patzakis6]. Mycobacterium tuberculosis and Brucella spp. are found less often [Reference Tali3, Reference Tekkok7]. Skin flora such as Staphylococcus epidermidis and other coagulase-negative staphylococci are associated with post-operative VO [Reference Hopkinson, Stevenson and Benjamin5, Reference Carragee8]. Fungi are rarely involved, with Candida accounting for 1–2% of VO cases [Reference Tali3, Reference Chhem9].
The annual incidence of VO in Europe has been estimated to be 2/100 000, but these estimates are based on studies covering limited areas and with few reported cases (32, 58 and 146, respectively) [Reference Hopkinson, Stevenson and Benjamin5, Reference Beronius, Bergman and Andersson10, Reference Jensen11]; the annual incidence in France is unknown. For these reasons, we undertook a study to estimate the national burden of VO in France. The aim of our study was to estimate the incidence of VO in France using a nationwide permanent hospital database launched in 1997 for medico-administrative purposes.
METHODS
Hospital-discharge database in France
A standardized discharge summary is collected for every hospital stay in France and categorized into a single medical or surgical diagnosis-related group (DRG) according to the diagnosis and procedures coded. The French DRG system (F-DRG) was developed from the US DRG system [Reference Fetter12]. The 10th edition of the International Classification of Diseases (ICD-10) has been used to code discharge diagnoses since 1996. The medical information contained in the database is anonymous and protected by professional confidentiality. Since 2001, an unique patient identification number has made it possible to link multiple hospital stays corresponding to a single patient without revealing his/her identity.
Initial database
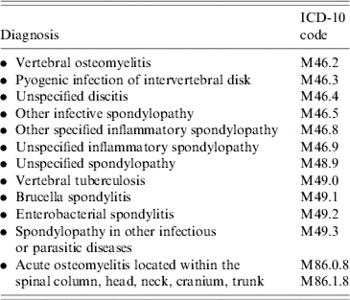
The Institut National de Veille Sanitaire (the National Institute for Public Health Surveillance), extracted the initial study database from the national public and private hospital database for the entire French population aged >1 year for the years 2002–2003. Only hospital stays corresponding to a primary or secondary diagnosis of specific ICD-10 codes used for coding VO were extracted (Table 1).
Table 1. Diagnoses, and 10th International Classification of Diseases codes, used for initial extraction of vertebral osteomyelitis hospitalizations, France, 2002–2003
Case definition
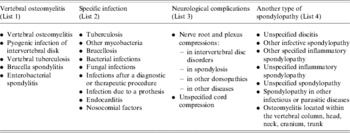
Three case definitions for VO, based on ICD-10 codes and their hierarchy in the discharge summary, were used (Table 2).
Table 2. List of 10th International Classification of Diseases codes used for case definition of vertebral osteomyelitis, France, 2002–2003
See Materials and Methods for the case definitions.
• Definite case. Hospital stay for which the primary diagnosis corresponded to one of the following ICD-10 codes: VO, pyogenic infection of intervertebral discitis, vertebral tuberculosis, brucella spondylitis, enterobacterial spondylitis (Table 2, list 1).
• Probable case. Hospital stay for which the primary diagnosis corresponded to a specific infection (Table 2, list 2) or a specific neurological complication (Table 2, list 3) identified by an ICD-10 code, in combination with one of the five ICD-10 codes included in the ‘definite case’ definition, coded as a secondary diagnosis.
• Possible case. Hospital stay with an unspecific spondylopathy (Table 2, list 4) in combination with a specific infection code (Table 2, list 2), regardless of whether they are a primary or secondary diagnosis.
The subsequent analysis includes only hospital stays matching one of these three VO case definitions.
Analysis of VO hospital stays
The following information was analysed for each VO stay: case definition, hospital status (public vs. private), type of admission and discharge, source and destination, month of discharge, length of stay (including intensive care), place of residence (geographic codes), place of hospitalization, and DRG-informative surgical procedure performed (i.e. procedures used to guide the categorization into a specific DRG group).
Analysis of VO patients
A patient database was created by using the unique patient identification numbers (linkage procedure). Only hospital stays fulfilling the case definition and where a match could be made with a unique patient identification number were used. In this way, the number of distinct patients and their management over the two years could be reconstituted and analysed. To compensate for the hospital stays excluded because of faulty linkage, extrapolation was performed to estimate the total number of patients hospitalized for VO per year.
The annual incidence of VO (definite, probable and possible cases) was calculated for the overall population, as well as by age, sex and region. The population data were derived from the 1999 census. Annual estimates at 1 January are available for the years 2002 and 2003.
The following information was analysed for each patient: sociodemographic data, number and total length of hospital stays, coded microorganism and comorbidities.
Validation of case definition
We estimated the positive predictive value of the case definition by analysing the complete medical records of VO cases registered in three regional university hospitals (Nantes, Poitiers and Tours) located in three of the 22 French regions. Anonymity was lifted at local hospital level.
Statistical analysis
The descriptive statistical analyses were carried out using SAS for Windows version 8 (SAS Institute Inc., Cary, NC, USA). Proportions were compared using the χ2 test; 95% confidence intervals (CI) of relative risks (RR) were calculated with Taylor series expansion.
RESULTS
Analysis of VO hospitalizations
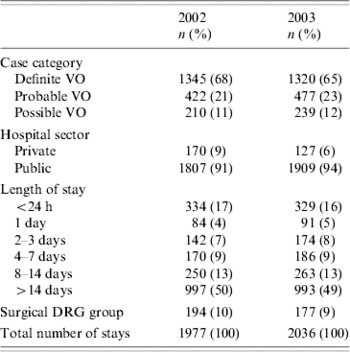
About 16 million hospitalizations occurred in France each year. Using the ICD-10 codes listed in Table 1, 8584 hospitalizations were identified as potentially attributable to VO. From this initial study database, 4013 hospital stays (47%) met the case definition for VO, 1977 in 2002 and 2036 in 2003. There were 67% definite, 22% probable and 11% possible cases over the two years combined (Table 3).
Table 3. Description of hospital stays for vertebral osteomyelitis, France, 2002–2003
VO, Vertebral osteomyelitis; DRG, diagnosis-related group.
More than 90% of hospitalizations for VO were in public hospitals. The mean length of stay was 25 days in 2002 (range 1–392 days), and 24 days in 2003 (range 1–142 days), with a median duration of 14 days for the two years (Table 3). A total of 107 stays (3%) included time spent in an intensive care unit (ICU). The mean duration of time in an ICU was 11 days (range 1–78 days, median 5 days). In total, 371 stays (9%) were categorized as a surgical DRG. Among the various procedures coded in surgical or not surgical DRG, half of them were related to spinal column and/or subcutaneous tissues: 408 spine surgery (75%), 66 evacuation of infectious collections (12%), 50 spine biopsy (9%), 20 spine decompression (4%).
The geographical distribution of hospitalizations and patients' place of residence was roughly the same, suggesting that patients were not travelling between regions for care. The largest number of hospitalizations for VO in 2002–2003 occurred in Ile de France (893, 22%), which includes Paris and its suburbs, and in Nord Pas de Calais (447, 11%). No seasonality was noted (130–180 stays each month).
Analysis of VO patients
In the initial study database, 93% of the hospitalizations in 2002 and 84% in 2003 could be perfectly linked to a patient, i.e. the hospital stay(s) could be linked to a single patient. Hospitalizations for VO that could not be linked to a patient were excluded from further analysis.
Application of the case definition to the well-linked hospitalizations showed that 2519 patients were hospitalized for VO, 1326 in 2002 and 1193 in 2003. Over the two years combined, there were 64%, 24% and 12% cases of definite, probable and possible VO, respectively. Seventy-five percent of patients were hospitalized only once, 15% had a second hospital stay, and 10% had three or more stays. The information collected did not allow us to determine why patients needed several hospitalizations. The period of time between the first and last hospitalization for VO was <3 months in 76% of cases (range 1–16 months). The mortality rate for patients hospitalized with VO was 2·8% in 2002 and 3·5% in 2003.
Hospitalization with VO was more common in men, with a male:female ratio of 1·5 (1·4 in 2002 and 1·6 in 2003). The mean age of hospitalized patients was 59 years (median 63 years, range 1–98 years), with no difference over the two years of the study. Analysis by age group showed that there was no difference in VO hospitalization numbers by gender for children and people aged <20 years of age (Fig. 1). However, this group accounted for only 3% of all patients hospitalized for VO.
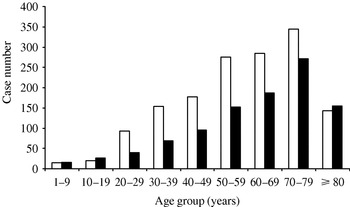
Fig. 1. Distribution of vertebral osteomyelitis cases by sex and age (years), France, 2002–2003. □, Male; ■, female.
After taking into account the number of hospitalizations excluded because of faulty linkage, we estimated that about 1400 patients (1422 in 2002, 1425 in 2003) are hospitalized for VO in France each year. This extrapolation was performed on the basis of the average number of stays of the initial database to obtain one patient corresponding to the case definition.
The annual incidence of VO (definite, probable and possible cases) was calculated in the population of well-linked patients. The overall incidence was 2·2/100 000 in 2002 and 2·0/100 000 in 2003, 2·6/100 000 in males and 1·7/100 000 in females. When only definite cases are included, the incidence was 1·5/100 000 in 2002 and 1·2/100 000 in 2003.
After extrapolation, the overall incidence was 2·4/100 000 in 2002 and 2003. Analysis of incidence by age group indicated predominance for males aged >20 years; this gender predominance increased steadily up to age 80 years (Fig. 2). The incidence of VO varied by region from 1·5 to 6·5/100 000. Differences by region were not statistically significant.
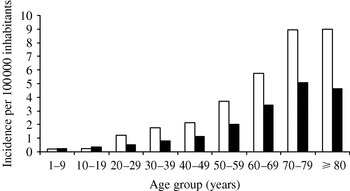
Fig. 2. Incidence of vertebral osteomyelitis by sex and age (years), France, 2002–2003. □, Male; ■, female.
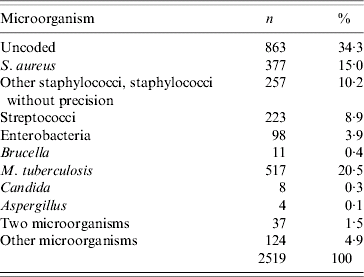
In 34% of cases, no information about the microbiological aetiology of the VO was recorded. In the remaining 1656 patients, at least one microorganism was coded. Staphylococcus spp. was recorded for 634 patients (38% of VO patients with a microbiological diagnosis coded), with S. aureus recorded for 377 patients (23% of VO), and other staphylococci or ‘staphylococci without precision’ for 257 patients (15%). Streptococci were coded for 223 patients (13·5%) and enterobacteria for 98 cases (6%). M. tuberculosis was found in 517 patients (31%) (Table 4). Among cases with a coded microorganism, the proportion of M. tuberculosis was significantly greater in Ile de France (56%) than in all the other regions (22%, P=0·001). In contrast, the proportion of VO cases caused by Staphylococcus spp. was significantly higher in other regions (44%) compared with Ile de France (23%, P<0·001). The incidence of vertebral tuberculosis in Ile de France (2·2/100 000) was significantly higher than in the other regions (0·49–100 000) (P<0·001). Patients aged <40 years were more likely to have vertebral tuberculosis than those over 40 (RR 2·7, 95% CI 2·39–3·08).
Table 4. Distribution of coded micro-organisms, in vertebral osteomyelitis, France, 2002–2003

Analysis of coded comorbidities showed that sepsis (septicaemia or bacteraemia codes), diabetes and endocarditis were associated in 27%, 11% and 9% of VO patients, respectively. Five percent of patients had cancer. More than 5% of VO cases had a concomitant urinary tract infection recorded, while spinal cord or nerve root compression was observed in more than 3% of cases. Four percent and 2% of patients also had a cardiac or orthopaedic prosthesis present. Chronic alcoholism and drug addiction were recorded for 3% and 1% of cases, respectively. One percent of overall VO patients were HIV-positive, associated with vertebral tuberculosis for 31% of them. The frequency of vertebral tuberculosis in HIV patients was not significantly higher than in non-HIV patients (20%). Only 1% of patients had at least two coded comorbidities. Post-operative complications, either infectious or ‘abnormal reaction’ following a medical or surgical procedure or prosthesis implant (excluding orthopaedic prosthesis), were reported in 5% cases of VO. Among VO patients with post-operative complications, 19% were coded as hospital-acquired infections.
Validation of case definition
To assess the specificity of the case definition used, 90 patient records from the regional university hospitals of Nantes (30 patients), Poitiers (32 patients) and Tours (28 patients) were reviewed. The diagnosis of VO was confirmed in 85 patients (98% in 60 definite cases, vs. 87% in 30 probable and possible cases, P=0·04), giving a positive predictive value of 94% (95% CI 87–98). The diagnosis of VO could not be confirmed in five patients: two patients with clinical suspicion of VO not confirmed by microbiological and radiological examinations, one spinal cord compression of non-infectious origin, one infectious endocarditis without VO and one patient lost to follow-up. This mild defect of specificity has a negligible effect on the incidence calculation (2·3/100 000).
DISCUSSION
VO is felt to be a relatively uncommon disease, although only a few limited-scale epidemiological studies have been reported [Reference Hopkinson, Stevenson and Benjamin5, Reference Beronius, Bergman and Andersson10, Reference Jensen11]. The F-DRG system was designed for economic and budgeting purposes [Reference Fetter12] and, for a long time, its application in epidemiology was limited by the fact that it was not possible to link multiple stays for a single patient. Since the creation of unique identification numbers in 2001, it has been possible to link stays to patients, and hence to estimate incidences at the national level [Reference Fosse13]. Using this system, we set out to estimate the incidence of VO in France, since, in France, this disease is primarily diagnosed and treated in hospital.
Different health-care professionals may select different ICD-10 discharge codes, or vary the position of the codes in the discharge summary, when coding for the same disease. This fact may affect the reliability of using ICD codes as proxies for diseases in surveillance studies or epidemiological research. In view of the potential complexity of different ways to code for VO, we created a case definition that favoured specificity over sensitivity. The robustness of the definition was confirmed by checking a sample of hospitalization discharges against complete medical records. In this validation sample, limited to three French regions, the positive predictive value of the case definition was 94%. However, the sensitivity of the case definition was not assessed.
Over the 2-year period of this study, only 90% of hospitalizations for VO could be linked to unique patients. An identical figure was observed in an epidemiological study of cancer in the Centre region of France over the same period (Agence régionale d'hospitalization, Région Centre, April 2006, unpublished data). Improving the ability to link hospital stays with individual patients, collecting data over a longer period of time, and closer attention to the quality of discharge coding should lead to better estimates of VO incidence in the future.
By extrapolating the estimates of VO incidence in linked patients to the entire number of hospitalizations for VO, we obtained an annual incidence of 2·4/100 000 in 2003–2004 in France. These results are consistent with data from Scandinavia and the United States [Reference Beronius, Bergman and Andersson10, Reference Jensen11]. Moreover, the sex- and age-specific incidences are comparable to those obtained by Beronius et al. in Göteborg, Sweden [Reference Beronius, Bergman and Andersson10]. The male predominance may be explained by a higher frequency of comorbidities in men aged >60 years. However, it is difficult to draw any firm conclusions about the impact of comorbidities on VO from these data as comorbidities that do not increase the rate of hospital stays are underreported in the F-DRG system (S. Baron, personal observation). Therefore, stronger conclusions can be drawn about diabetes complications or endocarditis than about intravenous drug addiction or obesity (no increase in charge), however, medical records have not been checked to confirm this hypothesis. Endocarditis was reported in 9% of patients hospitalized for VO, which is in accordance with some studies (2–10%) [Reference Nolla14, Reference Perronne15] although lower than the rate of 24% recently observed in another study [Reference Roblot16]. This study confirms that VO is a disease with appreciable morbidity and mortality. Three percent of hospitalizations included time spent in ICUs, and 3% of cases died during their hospitalization. One in 10 discharge summaries for VO included DRG-informative surgical procedure codes. Spinal surgery, biopsies for diagnostic purposes and debridements are likely to be directly related to the management of VO; however, this assumption was not verified by reviewing medical records. Therefore, we do not know whether the surgical interventions preceded VO (post-operative VO) or were undertaken as diagnostic or therapeutic steps in the management of VO. Unless discharge summaries included the ICD-10 code of nosocomial factor (Y95) and/or specific codes of post-operative infections, it was not possible to differentiate spontaneous from post-operative VO with these data. However, hospital-acquired infections are often underreported in hospital-discharge data in France [Reference Fourquet17].
Microbiological aetiology for VO is poorly documented by the F-DRG system, as microbiological procedures are not well-coded in the discharge summary and the coding of microorganisms has no impact on the charge for the hospital stay. The microorganism was not specified in 34% of VO cases, a proportion that may be attributed to the difficulty in isolating the causative microorganism in 30–40% of VO cases [Reference Khan, Vaccaro and Zlotolow2, Reference Hopkinson, Stevenson and Benjamin5, Reference Beronius, Bergman and Andersson10, Reference Wang18] and/or to faulty coding. Further investigations are essential to ascertain a more complete understanding of the microbiology of VO. The analysis of coded microorganisms showed a predominance of Staphylococcus spp. (38%), a finding consistent with the literature [Reference Khan, Vaccaro and Zlotolow2, Reference Tali3, Reference Jensen11]. Although our data demonstrated a predominance of Staphyloccus spp., we did not find a predominance of S. aureus as reported in the literature. This difference is probably due to imprecise coding practices, with S. aureus being coded using the imprecise Staphylococcus spp. code.
Tuberculosis was the second most common organism identified in VO, in line with the reported frequencies of 20–40% in different studies in Western countries [Reference Beronius, Bergman and Andersson10, Reference Perronne15, Reference Pertuiset19]. Our findings are also fully compatible with data obtained from compulsory reporting of tuberculosis in France [Reference Che, Campese and Decludt20, Reference Che and Bitar21]. The 2002 incidence of tuberculosis in Ile de France was 27·1/100 000, while it was 6·7/100 000 in other regions of France. In 2002 and 2003, about 300 cases of bone tuberculosis per year were reported to the compulsory reporting system (Institut de Veille Sanitaire, unpublished data, 2002). Our finding of 517 cases of vertebral tuberculosis in 2002–2003 would represent 86% of bone tuberculosis cases for those years. Patient origin (country of birth), a major risk factor for tuberculosis [Reference Wang18, Reference Barriere22], is not collected in hospital-discharge data. In France, tuberculosis tends to be concentrated in Paris and the Ile de France region where one out of six inhabitants is foreign-born, as in London [Reference Antoine, Maguire and Story23] and other large cities in Western Europe. In 2003, the incidence of tuberculosis in Ile de France was 3·5 times higher than in the rest of France, while the incidence of tuberculosis in foreign-born individuals was 13 times higher than in French-born individuals [Reference Che and Bitar21]. However, it should be borne in mind that it is not possible to establish with certainty whether the coded microorganism was the causative microorganism in all cases of VO.
In conclusion, the incidence of VO in France is estimated to be 2·4/100 000, which is in agreement with estimates available in other Western countries [Reference Beronius, Bergman and Andersson10, Reference Jensen11] The findings from this nationwide study are consistent with VO data reported in the literature and information obtained from the compulsory reporting of tuberculosis in France. The use of the F-DRG system has some methodological limitations and potential biases, but it nevertheless allowed us to estimate the burden of hospitalization from VO in France. By comparing the case definition based on ICD-10 coding with the information contained in the medical records, it was possible to demonstrate that the case definition used had a reasonable positive predictive value. This validation of the case definition is an important step in when using DRG codes for the surveillance of any rare disease whose diagnosis and coding are complex. Concerning microbiological aetiologies and risk factors, hypothesis suggested by hospital-discharge database analysis should in turn to be demonstrated with smaller focused studies.
ACKNOWLEDGEMENTS
Thanks are due to P. Ingrand, Ph.D., and F. Roblot, Ph.D., CHU de Poitiers, and P. Lombrail, Ph.D., CHU de Nantes, for their general support.
DECLARATION OF INTEREST
None.