Minimum ten-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization
Introduction
Degenerative lumbar spondylolisthesis can be encountered with degeneration of the lumbar motion segment (1). Typically, it causes spinal stenosis leading to neurogenic claudication or pain in the buttock, thigh, and leg (2). Surgery is indicated if conservative treatment fails and quality of life is progressively impaired (3,4). General objectives of surgical treatment are to decompress the spinal canal, prevent further slip, stabilize the segment and correct lumbosacral kyphosis in order to relieve back and leg pain and to reverse neurologic deficits. Classic surgical treatment consists of decompression to relieve radicular symptoms and neurogenic claudication (5-7). Fusion is added to prevent progression of spondylolisthesis. Several studies have shown that the combination of decompression and fusion significantly improves patient outcome compared with decompression alone (8-12), As long as Mulholland et al. (13) have hypothesized that abnormal load transmission is the principal cause of pain in osteoarthritic joints, in contrast to spinal fusion the dynamic stabilization system was created. The rationale for the dynamic stabilization system is to alter mechanical loading by unloading the disc but preserving lumbar motion in contrast to fusion (14). The Graf ligamentoplasty was the first commonly used posterior pedicle-screw-based non-fusion system (15-17). The guiding principle of that system was to stabilize spine in extension using pedicle screws connected by a non-elastic band. However, it increased the load over the posterior annulus, caused lateral recess and foraminal stenosis and was therefore only modestly successful (15).
As a result of the issues with Graf ligamentoplasty in 1994 the Dynesys system was developed (18). It is based on pedicle screws connected with a polyethylene cord and a polyurethane tube reducing movement both in flexion and extension (19,20).
The two and four years results of the first 26 patients after stabilization of single level degenerative spondylolisthesis of the years 1999 and 2000 were published in 2006 and 2008 respectively (21,22) and demonstrated a low rate of postoperative instability of 5% and a high overall patient satisfaction of 95%.
In the existing studies, dynamic stabilization was used for various indications with contradictory results, not allowing a conclusion if dynamic stabilization can meet the high expectations. Non-randomized studies reported promising results (20,23,24). To our knowledge, only one study with long-term surgical results of dynamic stabilization with a follow-up of up to 11 years (mean 7.2 years) has yet been published (25).
The aim of our prospective observational cohort study is to evaluate whether dynamic in situ stabilization for single level degenerative spondylolisthesis with the Dynesys System (Zimmer Spine, Minneapolis, MN, USA) without bone grafting provides enough stability to maintain significant clinical improvement and prevent progression of spondylolisthesis at long-term follow-up.
Methods
Patient selection
Between February 2000 and November 2003, a total of 127 patients with symptomatic lumbar spinal stenosis associated with degenerative spondylolisthesis grade I and II at a single or double level unresponsive to adequate non-operative treatment were operated in our hospital and underwent a lumbar decompression and stabilization with the Dynesys system of one or more levels.
In order to be included in the current study, the patients had to meet the following criteria: symptomatic lumbar spinal stenosis associated with degenerative spondylolisthesis grade I and II at a single level unresponsive to adequate trial of nonoperative treatment. All patients suffered from spinal claudication with leg pain with or without back or buttock pain. All patients underwent preoperative functional myelography and/or magnetic resonance imaging. In all patients, decompression was performed and the olisthetic segment was stabilized in situ with Dynesys (Zimmer Spine, Minneapolis, MN, USA) without adding any bone grafting material.
Patients with lytic spondylolisthesis, patients with degenerative spondylolisthesis of more than 1 level and patients with prior lumbar fusion were excluded.
Operative technique and postoperative care
Our operative technique has been described previously (21,22). Patients were operated under general anesthesia in prone position. Decompression of the stenotic levels was performed through a midline open approach. The dura was exposed and a laminotomy was carried out as far as needed to achieve proper decompression. In no case a laminectomy was necessary and the bony continuity of the lamina from left to right was always preserved. Most of the decompression was performed by undercutting. If needed, foraminotomy was additionally performed without resecting the isthmus. After adequate decompression, Dynesys pedicle screws were introduced under fluoroscopic control. The olisthetic segment was slightly distracted and the Dynesys system was placed under compression as described by Dubois et al. (26). No attempt was made to reduce the spondylolisthesis.
All patients were allowed to get up the first day after surgery and wore a lumbar orthosis for 12 weeks. After 3 months, patients were allowed to return to their normal activities without restriction.
Clinical outcome measures
The patients were reviewed after a minimum follow-up of 10 years by an independent spine surgeon who was not involved in the initial treatment. The following data were collected and compared with preoperative data: location of pain, intensity of pain according to the visual analog scale (VAS) (0 to100), neurologic symptoms, walking distance (WD), finger floor distance (FFD) to assess lumbar mobility, pain medication, complications caused by the operation, subsequent spinal surgery. The specific questionnaire for patients with lumbar spine diseases is the lumbar spine outcome assessment instrument of the North American Spine Society (NASS lumbar element), which was used in the validated German versions (27). The NASS Patient Satisfaction Index was used to assess treatment satisfaction: patients were asked “would you have the same treatment again for the same outcome?” and they could answer: definitely yes, probably yes, not sure, probably not, or definitely not (26).
Radiologic outcome measures
Plain radiographs (AP and lateral standing) and functional radiographs with flexion and extension views were obtained. On plain radiographs, the following variables were measured: spondylolisthesis grade (%), the segmental angle. On flexion-extension radiographs, the segmental angle of the stabilized segment was measured according to Cobb. In addition, any anterior or posterior translation was noted to detect instability. Increases of more than 5° or 3 mm are stated as significant (28). Implant failure, such as screw loosening or breakage, were noted. Finally, degenerative changes at adjacent levels were evaluated. Ten-year results were compared with preoperative data.
All radiographs were independently evaluated by two board certified spine surgeons who were blinded as to patient data and date of follow-up.
Statistical analysis
The clinical and radiological results were analyzed using Student t-test and Pearson (R1) and Spearman correlation coefficients (R2). The level of significance was set to 0.05. All statistical analyses were carried out with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
General data
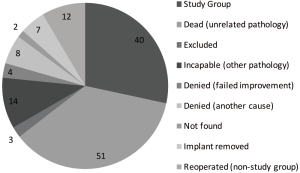
A total of 36 patients (30 females and 6 males) with a mean age at the operation time point was 66.53 (range, 47–80). 15 patients did not meet our inclusion criteria (i.e., 2 level stabilization), 50 patients had died at time of follow-up, 21 denied follow-up or could not be evaluated clinically because they were institutionalized elsewhere, 2 have moved out of the country, 3 could not be found.
Clinical findings
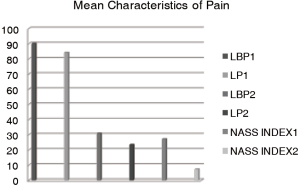
On average, patients have had symptoms for 18.83 months (range, 3–120; SE −3.92, SD −23.51). The mean VAS for low back pain (LBP) preoperatively and leg pain (LP) preoperatively was 90.17 (range 70–100; SE – 1.73, SD −9.14). And 83.66 (range, 0–100; SE −4.46, SD −24.01) respectively (Figure 1). The preoperative VAS data of LBP and LP could not be obtained for 7 and 5 patients respectively. The mean WD was 293 m (range, 5–1,500 m; SE −66.02, SD −349.37). The mean preoperative WD could not be attained by 7 patients. The mean FFD was 8.42 cm (range, 0–35; SE −2.27, SD −10.89). It could not be identified for 13 of the patients. Twelve patients (33.33%) had impaired sensation in terms of hypoesthesia, and 4 patients (11.11%) with diminished muscle strength (grade M4/5) according to affected level of the stenosis lesion. Nobody had acute bladder- or colon disturbances preoperatively.
Radiological findings
All the examined patients demonstrated spinal stenosis in the magnetic resonance imaging and/or myelography. Thirty-two patients (88.89%) had degenerative spondylolisthesis at the L4/L5 level. The mean listhesis grade due to Meyerding was 25.23% (range, −14% to 50%; SE −1.44, SD −6.28).
Perioperative data
There were no intraoperative complications. Postoperative complications included a deep vein thrombosis in one patient without pulmonary embolism.
Follow-up data
Clinical findings
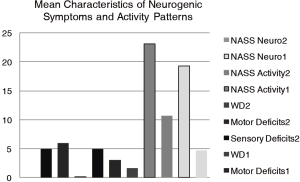
Mean follow-up was 10.78 years (range, 9–13 years). The mean VAS for LBP and LP decreased significantly to 30.7% (range, 15–100; SE −5.18, SD −30.67, P<0.05) and 19.58% (range, 0–95; SE −4.74, SD –28.07, P<0.05) combined with NASS-Pain scores respectively (Figure 1). The WD improved to 1,625 m (range, 0–3,000 m, SE –213.92, SD −1,265.6, P<0.05). The mean FFD was 8.82 cm (range, 0–30, SE −1.6, SD –9.04). There were 7 patients (19.44%), who noticed improvement but still had decreased sensation in their legs compared to preoperatively. One patient did not see any improvement in terms of sensation at follow-up. There were 8 patients (22.22%) with dermatome specific sensory deficits preoperatively, which were completely disappeared at the follow-up time point. In 3 patients (8.33%) non-specific sensory deficit remained constant by the follow up examination. They were reoperated as well. In 2 patients (5.56%) non-specific sensory deficits appeared firstly after operation. There were 3 patients (8.33%) with diminished muscle strength (M 3/4) due to affected level of lesion. In one case the muscle weakness manifested and remained stable after hip replacement surgery. Another two patients had no pathological findings preoperatively.
The mean values of pre- and postoperative sensory and motor deficits, as well as mean values of WD in kilometers pre- and postoperatively combined with NASS neurogenic symptoms and activity subscores are shown in Figure 2.
Five patients (13.9%) required reoperation. In one case (2.8%) patient had undergone double decompression at the same level without implant failure. One patient (2.8%) required decompression with Dynesys in 2002 at the same level L3/L4 and another decompression in 2004 at the level L4/L5. One patient (2.8%) had redecompression at the level L3/L4 and additional Dynesys at the same level without an implant failure at the level L4/L5. One patient (2.8%) required redecompression at the level L2–L4 and additional Dynesys at L3–L5 with an implant failure at the level L4/L5. One patient (2.8%) was reoperated with additional Dynesys at the level L5/S1 without an implant failure at L4/L5.
Quality of life questionnaire
The mean values of pain subscores due to NASS Pain Index1 (for the low back pain) was 26.49% (range, 0–75; SE −3.97, SD −23.15) and NASS Pain Index2 (for the leg pain) was 7.24% (range 0–30; SE −1.16, SD −6.76). The mean values of neurologic subscores due to NASS Neuro Index1 (due to LBP2) was 19.11% (range, 0–60; SE −3.15, SD −18.34) and Neuro Index2 (due to LP2) −4.57% (range, 0–14.12; SE −0.75, SD −4.35). The mean values of NASS Activity Index1 (due to LBP2) was 21.66% (range, 0–75, SE −2.88, SD −16.77) and NASS Activity Index2(due to LP2) was 10.15% (range, 0–27.06; SE −1.3, SD −7.63) respectively. The mean values of NASS activity and neurogenic symptoms subscores are also presented in Figure 2.
Radiological findings
Plain and functional radiographs of 36 patients were obtained at FU. The mean listhesis grade in neutral position was 11.07% (range, 0–25; SE – 1.77, SD −8.88), in reclination made up 11.5% (range, 0–25; SE – 1.27, SD −8.33) and the listhesis grade in inclination was 11.55% (range, 0–25; SE −1.32, SD −8.89). There were 2 asymptomatic screws that showed radiolucent lines in terms of loosening. No screw breakage was documented. In one case the implant failure (due to screw loosening) was noted and the patient had undergone the explantation and was provided with the same stabilization device. The average Cobb angle was 9.73° (range, 0–26; SE −1.28, SD −8.11). There were 5 patients (12.8%) with documented vertebral fractures treated conservatively.
In 3 patients (8.33%) there were no radiological changes in the operated and adjacent segments. There were 13 patients (36.11%) with progressing degenerative osteochondrosis in the adjacent segments, and 4 patients (11.11%) with progressing listhesis at the adjacent segments. Eight patients (22.22%) had both degenerative osteochondrosis and listhesis of the adjacent segments.
Correlations
Additionally, to our results, the correlations of different patterns were tested. Statistically significant correlations were observed among LBP and LP pre- and postoperatively (P=0.001/P=0.04) and LBP and LP pre- and postoperatively and NASS Pain (P=0.03) and Neuro Index (P=0.03), among LBP and LP and satisfaction level (P=0.01) and consumption of analgesics (P=0.03) at FU. The statistically significant correlations among patient’s age and FFD as well as the average listhesis grade preoperatively were obtained.
Additionally, the statistically significant correlations were noted between WD and NASS low back pain (P=0.002), Activity (P=0.004/P=0.002), Neuro Index1 (P=0.002) and satisfaction level (P=0.005), consumption of analgesics (P=0.01) at FU time point FFD showed significant correlations between NASS Neuro Index1/2 (P=0.03/P=0.04), consumption of analgesics (P=0.04), adjacent segment degeneration (ASD) (P=0.004) and motor deficits (P=0.04) at FU.
There were good to strong correlations among NASS Pain Index1 and NASS Activity1/2 (P=0.0001/P=0.001), and NASS Pain Index2 and NASS Activity1/2 (P=0.0001/P=0.005), and NASS Pain Index1 between Neuro Index (P=0.0001/P=0.003), satisfaction level (P=0.005), consumption of analgesics (P=0.01), as well as between sensory and motor deficits (P=0.03) at FU. NASS Neuro Index correlated well with NASS Activity Index (P=0.0001–0.0002/P=0.001–0.002) and consumption of analgesics (P=0.002–0.003), sensory (P=0.01–0.02) and motor deficits (P=0.007) at FU. And NASS Activity Index showed good to strong correlations between consumption of analgesics (P=0.02–0.05), satisfaction level (P=0.01–0.003) and motor deficits (P=0.04) at FU time point.
The analysis of other correlation-patterns showed statistically significant results only among satisfaction level and consumption of analgesics (P=0.01), as well as among sensory and motor deficits (P=0.04) at FU.
Discussion
Summarizing our study data, the statistically significant correlations among preoperative LBP and LP and postoperative pain patterns due to VAS and NASS Pain Index were observed, which emphasize substantial LBP and LP reduction through all those years (Figure 3); as well as the correspondence to satisfaction level and the use of analgesics.
The patient’s age was tested due to correlation within many other patterns; nonetheless it was only statistically significant related to the use of analgesics.
The mean WD preoperatively increased compared to FU, whereas the change was not statically significant. On the contrary, the mean WD at FU produced strong correlation with the NASS patterns; it even affected the satisfaction level and the consumption of analgesics at FU.
As well as, sensory and motor deficits reduced to 14% and 5% respectively in the patient population compared to preoperative results, though statistically insignificant. On the other hand, we had strong correlations among sensory and motor deficits and NASS Neuro Index at FU. The main factor producing essential influence on those characteristics was LBP and LP.
The mean FFD did no advance, however it was significantly correlated to many other characteristics, it could not be a measure of the mobility of the lumbar spine and therefore depends on hamstring muscles of the hips.
NASS pain, neurological deficit and activity characteristics showed statistically significant correlations with many patterns, per contra there was no statistical significance within radiological study patterns. The daily activity due to NASS lumbar questionnaire remained only moderate compared with the normal population (29).
The awaited significant changes among listhesis grade preoperatively and at FU, and the level of ASD could not be confirmed statistically and therefore shows minimal changes in the stabilized segment without signs of instability. Our results also corroborate the retrospective radiographic analysis by Cakir et al. (30).
The current study supplements our past studies with FU of 2 and 4 years (21,22). However, the study sample was not the same and because of incomplete data it had to be designed in a bit other way.
The mean pain on VAS decreased significantly too, though slightly better values were observed after 2 and 4 years of FU. In contrast to the previous studies there were only 37.5% and 50% of clinical subjects without back pain and claudication symptoms respectively. Thus, the reduction of neurogenic symptoms was detected; the difference was not as encouraging as in short-term and middle term trials. Consequently, mean WD improved immensely and remained the same even over 10 years of FU. Opposed to previous findings with 32% and actually 47.5% of our patient population were still on analgesics, however the comorbidity rate could be the most important factor maintaining this probable discrepancy. In our trial 8.5% of patients were more satisfied with the conducted surgery, though the more patients would not undergo the same procedure compared to the previous studies (21,22).
Absolutely different radiological findings suggesting of marked progression of ASD were retrieved from our study. In only 5% of clinical cases asymptomatic screw loosening occurred, however 12.5% of study population were additionally operated due to progression of ASD without an implant failure.
To compare our findings with the most similar long-term study by Hoppe et al. (25), even 66.7% of patients in their trial required no analgesics. Though 21% of patients were reoperated, 83% of patients were satisfied with the intervention and 92% would undergo the surgery again, which is a slightly better result in contrast to our study.
Analyzing the radiological outcomes, ASD was more than twofold progressive in our trial. Our result is overcoming other long-term trials (31-33), where symptomatic ASD reported to be between 15% and 35%. Range of motion at the instrumented segment was markedly reduced in both trials. The rate of screw loosening was slightly better; likewise, no implant loosening could be noted in our study. The implant failure was documented only in one case in both surveys.
Though literature addressing long-term results of dynamic stabilization devices is sparse, available data of various short- and middle-term trials could be reviewed.
Considering the alternative and primary applied posterior pedicle-screw device—Graf ligamentoplasty (15,19,23). A couple of short- and middle-term surveys by Grevitt, Gardner and Brechbühler showed promising results and concluded that good results were seen in patients with combination of minor disc degeneration and mild loss of intervertebral height, fixed back musculature and facet arthritis. Several other studies (13,34-36) demonstrated higher revision rates and distinct advantage of posterolateral fusion.
Series of short to middle follow-up studies reported very encouraging results with Dynesys, like in 83 cases of patients with variety of degenerative disorders reported by Stoll et al. (20), where no screw breakage could be noted, however 7 screw loosening and 9 implant failures occurred. It was postulated, that the lack of breakage might be due to the elasticity of spacers-cord combination. Bordes-Monmeneu, Hu and Lee et al. (37-39) announced good results over 2 years of follow-up concerning the quality of life with over 50% of improvement. The last study had even no implant failure cases and concluded that Dynesys with appropriate surgery indications might be a viable alternative to spinal fusion, which is actually also supported by aforementioned trials by Hoppe et al. (25), our previous (21,22) and current survey.
Yu et al. (40) compared the effectiveness of Dynesys opposed to PLIF in 35 and 25 patients respectively, where the Dynesys patients had a higher preservation of motion at operative levels as well as total range of motion. Additionally, they noted the Dynesys group showing a greater improvement in Oswestry Disability Index (ODI)and VAS and therefore concluding that Dynesys has been an acceptable alternative to PLIF within three years of FU.
Several trials announced negative results, likewise Würgler-Hauri et al. (41) described high complication rate including 4 broken and 2 misplaced pedicle screws within a total of 224 screws implanted in 12 months of FU. During this time, even 19% of patients required surgical revision. Grob et al. (42) assessed high reoperation rate (19%), however the Dynesys was implanted for a variety of indications, thus making firm conclusions impossible.
Some controversial studies regarding unusual indications appeared during that time. While Vaga et al. (43) outlined good results concerning ODI and VAS improvement, as well as glycosaminoglycans (GAG) concentration within instrumented levels and even marked GAG reduction of the noninstrumented levels.
Ko et al. (44) noted an overall radiographic evidence of loosening in 19.7% patients and 4.6% of screws. Though no adverse effect on clinical improvement while 16.6 months of FU could be proved.
Di Silvestre et al. reported (45) outcomes in patients with mild degenerative scoliosis and Cobb angle of 16.9 degrees who underwent Dynesys with laminectomy. ODI, pain and Cobb angle improved considerably.
Our study had some limitations. Firstly, only 40 patients were eligible to process the current study group due to various reasons listed in Figure 3. Secondarily, the preoperative quality of life data in accordance with the NASS spine instrument were absent. Due to missing preoperative radiological data, the part of range of motion measures were not precise as awaited and the measurement of disc height could not be implemented, supported in our previous studies by Schaeren et al. (21,22).
Conclusions
Decompression and single level dynamic in situ stabilization with the Dynesys System demonstrate good clinical and radiological long-term results in elderly patients. It maintains enough stability to prevent progression of spondylolisthesis without the need for fusion surgery and its associated risks and morbidity. It does not, however, prevent adjacent segment disease. The rate of secondary surgeries is comparable to other posterior instrumentation devices.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was reviewed by the local ethical committee (Ethikkkommission beider Basel, No. V5-25.04.2012). Informed written consent was obtained from each participant.
References
- Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am 1975;57:467-74. [Crossref] [PubMed]
- Jacobsen S, Sonne-Holm S, Rovsing H, et al. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine (Phila Pa 1976) 2007;32:120-5. [Crossref] [PubMed]
- Herkowitz HN. Spine update. Degenerative lumbar spondylolisthesis. Spine (Phila Pa 1976) 1995;20:1084-90. [Crossref] [PubMed]
- Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: a 10- to18-year follow-up study. J Neurosurg 2000;93:194-8. [PubMed]
- Johnsson KE, Willner S, Johnsson K. Postoperative instability after decompression for lumbar spine stenosis. Spine (Phila Pa 1976) 1986;11:107-10. [Crossref] [PubMed]
- Katz JN, Lipson SJ, Larson MG, et al. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am 1991;73:809-16. [Crossref] [PubMed]
- Lombardi JS, Wiltese LL, Reynolds J, et al. Treatment of degenerative spondylisthesis. Spine (Phila Pa 1976) 1985;10:821-7. [Crossref] [PubMed]
- Bassewitz H, Herkowitz H. Lumbar stenosis with spondylolisthesis: current concepts of surgical treatment. Clin Orthop Relat Res 2001.54-60. [Crossref] [PubMed]
- Bridwell KH, Sedgewick TA, O'Brien MF, et al. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord 1993;6:461-72. [Crossref] [PubMed]
- Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 1991;73:802-8. [Crossref] [PubMed]
- Nork SE, Hu SS, Workman KL, et al. Patient outcomes after decompression and instrumented posterior spinal fusion for degenerative spondylolisthesis. Spine (Phila Pa 1976) 1999;24:561-9. [Crossref] [PubMed]
- Postacchini F, Cinotti G, Perugia D. Degenerative lumbar spondylolisthesis. II Surgical treatment. Ital J Orthop Traumatol 1991;17:467-77. [PubMed]
- Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J 2002;11 Suppl 2:S198-205. [PubMed]
- Nockels RP. Dynamic stabilization in the surgical management of painful lumbar spinal disorders. Spine (Phila Pa 1976) 2005;30:S68-72. [Crossref] [PubMed]
- Brechbühler D, Markwalder TM, Braun M. Surgical results after soft system stabilization of the lumbar spine in degenerative disc disease--long-term results. Acta Neurochir (Wien) 1998;140:521-5. [Crossref] [PubMed]
- Gardner A, Pande KC. Graf ligamentoplasty: a 7-year follow-up. Eur Spine J 2002;11 Suppl 2:S157-63. [PubMed]
- Grevitt MP, Gardner AD, Spilsbury J, et al. The Graf stabilization system: early results in 50 patients. Eur Spine J 1995;4:169-75; discussion 135. [Crossref] [PubMed]
- Anand N, Baron EM. Role of dynesys as pedicle-based nonfusion stabilization for degenerative disc disorders. Adv Orthop 2012;2012:218385. [PubMed]
- Schwarzenbach O, Berlemann U, Stoll TM, et al. Posterior dynamic stabilization systems: DYNESYS. Orthop Clin North Am 2005;36:363-72. [Crossref] [PubMed]
- Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J 2002;11 Suppl 2:S170-8. [PubMed]
- Schnake KJ, Schaeren S, Jeanneret B. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine (Phila Pa 1976) 2006;31:442-9. [Crossref] [PubMed]
- Schaeren S, Broger I, Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine (Phila Pa 1976) 2008;33:E636-42. [Crossref] [PubMed]
- Putzier M, Schneider SV, Funk JF, et al. The surgical treatment of the lumbar disc prolapse: nucleotomy with additional transpedicular dynamic stabilization versus nucleotomy alone. Spine (Phila Pa 1976) 2005;30:E109-14. [Crossref] [PubMed]
- Welch WC, Cheng BC, Awad TE, et al. Clinical outcomes of the Dynesys dynamic neutralization system: 1-year preliminary results. Neurosurg Focus 2007;22:E8. [Crossref] [PubMed]
- Hoppe S, Schwarzenbach O, Aghayev E, et al. Long-term outcome after monosegmental L4/5 stabilization for degenerative spondylolisthesis with the dynesys device. Clin Spine Surg 2016;29:72-7. [Crossref] [PubMed]
- Dubois G, der Germay B, Schaerer NS, et al. Dynamic neutralization: a new concept for restabilization of the spine. In: Spzalski M, Gunzburg R, Pope MH, editors. Lumbar segmental Instability. Philadelphia, PA: Lipincott, Williams and Wilkins, 1999:233-40.
- Pose B, Sangha O, Peters A, et al. Validation of the North American Spine Society Instrument for assessment of health status in patients with chronic backache. Z Orthop Ihre Grenzgeb 1999;137:437-41. [Crossref] [PubMed]
- Aota Y, Kumano K, Hirabayashi S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. J Spinal Disord 1995;8:464-73. [Crossref] [PubMed]
- Schaeren S, Bischoff-Ferrari HA, Knupp M, et al. A computer touch-screen version of the North American Spine Society outcome assessment instrument for the lumbar spine. J Bone Joint Surg Br 2005;87:201-4. [Crossref] [PubMed]
- Cakir B, Carazzo C, Schmidt R. Adjacent segment mobility after rigid and semirigid instrumentation of the lumbar spine. Spine (Phila Pa 1976) 2009;34:1287-91. [Crossref] [PubMed]
- Rahm MD, Hall BB. Adjacent-segment degeneration after lumbar fusion with instrumentation: a retrospective study. J Spinal Disord 1996;9:392-400. [Crossref] [PubMed]
- Throckmorton TW, Hilibrand AS, Mencio GA, et al. The impact of adjacent level disc degeneration on health status outcomes following lumbar fusion. Spine (Phila Pa 1976) 2003;28:2546-50. [Crossref] [PubMed]
- Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am 2004;86-A:1497-503. [Crossref] [PubMed]
- Hadlow SV, Fagan AB, Hillier TM, et al. The Graf ligamentoplasty procedure: comparison with posterolateral fusion in the management of low back pain. Spine (Phila Pa 1976) 1998;23:1172-9. [Crossref] [PubMed]
- Rigby MC, Selmon GP, Foy MA, et al. Graf ligament stabilisation: mid-to long-term follow-up. Eur Spine J 2001;10:234-6. [Crossref] [PubMed]
- Sengupta DK. Dynamic stabilization devices in the treatment of low back pain. Neurol India 2005;53:466-74. [Crossref] [PubMed]
- Bordes-Monmeneu M, Bordes-Garcia V, Rodrigo-Baeza F, et al. System of dynamic neutralization in the lumbar spine: experience on 94 cases. Neurocirugia (Astur) 2005;16:499-506. [Crossref] [PubMed]
- Hu Y, Gu YJ, Xu RM, et al. Short-term clinical observation of the Dynesys neutralization system for the treatment of degenerative disease of the lumbar vertebrae. Orthop Surg 2011;3:167-75. [Crossref] [PubMed]
- Lee SE, Park SB, Jahng TA, et al. Clinical experience of the dynamic stabilization system for the degenerative spine disease. J Korean Neurosurg Soc 2008;43:221-6. [Crossref] [PubMed]
- Yu SW, Yen CY, Wu CH, et al. Radiographic and clinical results of posterior dynamic stabilization for the treatment of multisegment degenerative disc disease with a minimum follow-up of 3 years. Arch Orthop Trauma Surg 2012;132:583-9. [Crossref] [PubMed]
- Würgler-Hauri CC, Kalbarczyk A, Wiesli M, et al. Dynamic neutralization of the lumbar spine after microsurgical decompression in acquired lumbar spinal stenosis and segmental instability. Spine (Phila Pa 1976) 2008;33:E66-72. [Crossref] [PubMed]
- Grob D, Benini A, Junge A, et al. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine (Phila Pa 1976) 2005;30:324-31. [Crossref] [PubMed]
- Vaga S, Brayda-Bruno M, Perona F, et al. Molecular MR imaging for the evaluation of the effect of dynamic stabilization on lumbar intervertebral discs. Eur Spine J 2009;18:40-8. [Crossref] [PubMed]
- Ko CC, Tsai HW, Huang WC, et al. Screw loosening in the Dynesys stabilization system: radiographic evidence and effect on outcomes. Neurosurg Focus 2010;28:E10. [Crossref] [PubMed]
- Di Silvestre M, Lolli F, Bakaloudis G, et al. Dynamic stabilization for degenerative lumbar scoliosis in elderly patients. Spine (Phila Pa 1976) 2010;35:227-34. [Crossref] [PubMed]