
MIS approaches in the cervical spine
Introduction
Innovative technology and techniques have revolutionized minimally invasive spine (MIS) surgery within the last decade. Cervical pathologies, which are unique in their approaches, indications, and risks, have taken longer to garner support with MIS treatment strategies compared with thoracic and lumbar diseases. The smaller working space, reduced mobility and increased sensitivity of the cervical spinal cord, and the catastrophic risk of cervical spinal cord injury have factored in this hindrance. Anterior cervical approaches can be complicated by great vessel anatomy, dysphagia, and phonation issues, whereas posterior approaches can result in severe postoperative neck pain from muscular dissection and delayed iatrogenic deformities and instability. Fusion and non-fusion procedures also play a critical role in surgical decision making when considering cervical MIS options.
Despite this, advances in cervical MIS surgery for certain favorable anatomic pathologies have gained traction in recent years. From an anterior perspective, approaches to the craniovertebral junction or upper cervical spine through a trans nasal route as opposed to transoral approaches have become increasingly described and validated. From a posterior perspective, minimally invasive options for decompressive laminectomy and foraminotomy via tubular retractors have been reported. Finally, traditional lateral mass screw and rod constructs are now being contrasted with percutaneous facet joint cage implants as a posterior arthrodesis technique. In the following review, we describe these novel techniques and their role in patient management, as well as discussing the increasing role of 3-dimensional (3D) navigation in surgical planning and execution.
Endoscopic endonasal odontoidectomy
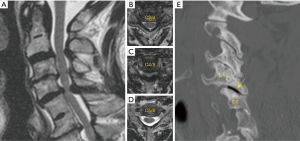
Ventral abnormalities of the odontoid can lead to compression of the cervicomedullary junction and upper cervical spine. These conditions can develop secondary to trauma, infection, or inflammatory conditions such as rheumatoid pannus (1). They can also be seen in congenital disorders resulting in basilar invagination from a retroflexed odontoid, as seen in those with Down syndrome, achondroplasia, or Type 1 Chiari malformation (2,3). Traditionally, these lesions were treated with anterior decompression via transoral techniques. However, this approach has a high complication rate due to infection and velopharyngeal insufficiency (4,5). Patients are also at higher risk of requiring tracheostomy and non-oral methods of nutritional supplementation due to prolonged time intervals to extubation and recovery of the oral-enteral pathway (4,5). In light of this, advances in endoscopic technology have provided an alternative, endonasal approach for odontoidectomy in patients with amenable anatomy in conjunction with occipitocervical fusion (OCF) (Figure 1).
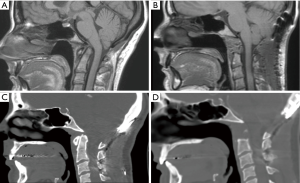
OCF is required as the first part of this procedure since resection of the anterior arch of C1, the odontoid, and transverse ligament result in severe instability at the craniovertebral junction. This can be performed in the same day or as a staged procedure over 2 days. The rhino palatine line is a reliable predictor for determining if a patient is a candidate for this approach. This is determined using a midsagittal image and drawing a line from 2/3rds the distance between the rhinion and the anterior nasal spine, back to the posterior nasal spine of the palatine bone, then back to the spine (6). When the odontoid is located superior to his line, the extended endoscopic endonasal approach is feasible.
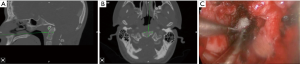
3D navigation is essential to this procedure since there are no reliable landmarks for incising the nasopharyngeal fascia (Figure 2A,B). Once this is completed, navigation can help guide the depth of drilling for bony resection (Figure 2C). Postoperatively, as contrasted with transoral approaches, patients demonstrate earlier time to extubation and less dysphagia (7). Furthermore, when indicated, pathologies that involve the skull base (such as a dorsal clival lip) can be more easily reached (8).
Posterior cervical foraminotomy (PCF)
Earliest reports of PCF were described in the 1950s and minimally-invasive techniques were first reported in the early 2000s (9). Ideal candidates possess single level disease with unilateral radiculopathy, often due to foraminal soft disc fragment, bone spur, or synovial cyst (10-12). In patients with myelopathy, central stenosis, instability, and kyphotic deformity, this procedure is contraindicated since it can exacerbate these conditions. Preoperatively patients should have flexion/extension imaging to confirm there is no dynamic instability and MR images should be carefully reviewed to exclude pre-existing contralateral facet joint pathology. The minimally-invasive technique has shown comparable outcomes to the open approach with more rapid functional recovery, shorter inpatient stays, and earlier return to work (13,14).
Studies comparing ACDF and PCF for unilateral cervical radiculopathy have demonstrated equivalent clinical outcomes at an average of 12–43 months after surgery based on improvements in neck and arm pain (15-17). A report by Wang et al. demonstrated that 5% of patients that had posterior cervical foraminotomy went on to have an ACDF at the index level at an average of 31 months postoperatively (18). Another retrospective analysis of a prospectively-collected cohort undergoing PCF or discectomy demonstrated a 1.1% risk per year of requiring fusion at the index level and 0.9% annual risk of adjacent segment disease requiring surgery (19). These comparable outcomes with relatively low risk are also favorable due a decreased risk of dysphagia, recurrent laryngeal nerve, and motion preservation when faced with the option of performing ACDF. On the contrary, there is significantly more muscle dissection involved for PCF (9). C5 palsy is also more common when performing PCF than anterior foraminotomy specifically at the C4/5 level, with rates of 14% having been reported in large series, especially in patients of older age (20). While relatively low risk, delayed instability can occur. Furthermore, dual treatment for concomitant deformity or mechanical neck pain cannot be adequately addressed.
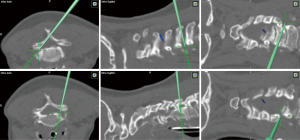
The technical aspect of the procedure involves positioning the patient prone with rigid head fixation. A small paramedian incision is made over the localized level and side, then sequential dilators and the corresponding sized tube is placed. Typically, the procedure can be successfully performed through a 16 mm tubular retractor. 3D navigation can be used for precise localization of the incision and docking site as well as the maximal safe extent of the foraminotomy (Figure 3). Using microscopy, the lateral aspect of the lamina and medial third of the facet joint is removed using a high-speed burr. Studies have shown that removing greater than 50% of the facet joint can result in greater risk of delayed mechanical failure (21,22). Once the lateral aspect of the thecal sac is identified, Kerrison rongeurs are used to follow the exiting nerve root laterally and widen the foraminotomy. In cases where there is a soft foraminal disc herniation, the nerve must be retracted cranially to allow room for the discectomy to be performed using micro-pituitary rongeurs. The contralateral foramen can also be accessed when performed in conjunction with an “over the top” laminotomy in patients with bilateral radicular pain and foraminal stenosis. Of note, brisk venous bleeding can often be encountered during this procedure from the epidural venous plexus. Head elevation by reverse Trendelenburg, thrombin-soaked gel foam, and hemostatic matrix foams with pressure are usually suffice to obtain hemostasis.
Cervical unilateral laminotomy for bilateral decompression (ULBD)
Traditional open cervical laminectomy is associated with risks such as delayed kyphosis and instability (23-25). In 1997, the concept of bilateral spinal decompression via a unilateral approach was first described in lumbar surgery and subsequently minimally invasive options to perform this technique were published (26-28). This approach has now become a key surgical principal of MIS surgery and has been applied to all levels of the spinal column (29,30). Patients with cervical spondylotic myelopathy without evidence of instability can be treated for minimally invasive cervical decompression via a ULBD “over the top” technique (Figure 4). This procedure is advantageous to open approaches due to reduction in blood loss, muscle disruption, and bony removal. While biomechanical and clinical studies comparing this technique to traditional open approaches with long term outcomes have not reported, comparative literature from other segments of the spine suggest there is a reduced risk of post-laminectomy kyphosis, secondary fusion requirements, infections, postoperative narcotic dependency, and hospital stay. This approach is especially beneficial for obese and elderly patients in whom an open procedure would require more exposure and be considered more risky (31,32).
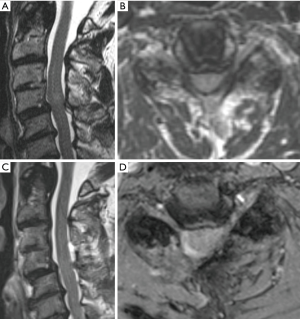
From a technical perspective, the patient is positioned in the prone position and the neck is neutral or slightly flexed. 3D navigation is used to localize the exact entry point, and a 2.5–3 cm incision is made over the affected level. Sharp dissection or monopolar cautery is continued until the fascia is identified. Dilators of increasing size are then passed over another to expand the corridor. 16–18 mm tubular retractors are typically used once the appropriate depth is determined. The triangle formed by the caudal lamina and lamino-facet junction should be identified after removing any remaining soft tissue. The caudal aspect of the lamina should be drilled in a cranial direction until the cranial attachments of the ligamentum flavum are identified. The operating table should be rotated and the tubular retractor should be tilted to have an appropriate trajectory for performing the contralateral decompression, undercutting the spinous process (Figure 5). The ventral surface of the contralateral lamina is drilled away taking care to not exert significant downward pressure on the spinal cord when retracting down with a Frazier suction. To strip the ligamentum flavum form its bony attachments a ball-tip probe is used. Kerrison rongeurs are used to complete the ligamentum resection caudally and in the medial and lateral directions. Finally, the surgeon should inspect the decompression by tilting the retractor in all directions. 3D navigation is useful in guiding the extent of the contralateral and ipsilateral laminotomy (Figure 6).
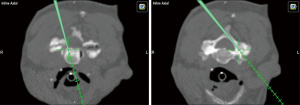
Posterior percutaneous facet joint fusion
Facet distraction was first described by Goel et al. as a treatment for cervical spondylotic radiculopathy and myelopathy (33-36). This concept has now progressed to percutaneously placed, posterior cervical facet joint cage implants (e.g., DTRAX; Providence Medical Technology, Inc, Pleasanton, CA) as an alternative posterior approach for cervical fusion compared with lateral mass fixation (37-39). A cadaveric study from 2016 showed that bilateral posterior interfacet cages provided similar cervical segmental stability compared with an open lateral mass instrumented fusion (40). Patient typically present with foraminal stenosis with radiculopathy, facet mediated pain, or pseudoarthrosis. This procedure can also be performed in conjunction with multi-level anterior decompression and fusion or adjacent level compromise following prior posterior fusion. Contraindications include metastatic or primary tumors involved the face joint or traumatic injury of the facet joint. Infection and those with high grade listhesis are also not eligible for this approach (34,39). For patients with significant medical comorbidities that require cervical decompression and stabilization and are otherwise high risk for traditional cervical lateral mass fusion, this procedure provides an attractive alternative (Figures 7,8).
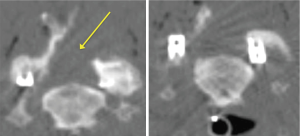
Initial reports have described significant improvements in mean Neck Disability Index (NDI) scores, SF-12v2, and pain visual analog scores (41) 2 weeks after surgery which persist up to a year later (39). Hardware failures and vertebral artery, spinal cord, or nerve root injuries are rare, however complication rates of up to 4.3% having been reported (39,42). A 2016 study demonstrated that radiographic fusion rate after 2 years follow up was 98.1% and there were no significant changes in overall or segmental kyphosis, device failures, implant lucencies, or surgical reinterventions (37). Another study showed that after 5-year follow-up, there were no instances of recurrent radicular symptoms (36). Additionally this approach has a faster recovery time, shorter hospital stay, and less blood loss (42).
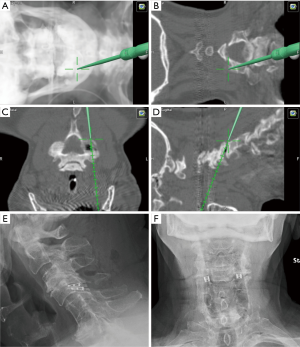
In planning for the procedure, SSEP and MEP neuromonitoring should be employed. The patient is placed in the prone position and the head is placed in rigid fixation. Using 3D navigation (or fluoroscopy when navigation is not available), the medial and lateral aspect of the facet are identified (Figure 8A,B,C,D). An access chisel is used to enter joint in a medial to lateral trajectory and is collinear to the facet joint. Medial positioning of the implants could result in nerve root injury. The decortication trephine is slid down over the chisel onto the facet and decorticated the joint capsule. The inside of the facet joint is reamed out with a pineapple-tipped decortication burr. After the burr is removed, a cage filled with graft material is inserted and unlocked. Finally, the bone screws are introduced to lock the implant into the inferior articular facet (Figure 8E,F). Every instrument is removed except the guide tube. Through the guide tube bone graft is delivered in to the joint. The guide tube is removed, the wound is irrigated and closed in usual fashion.
Navigation in cervical MIS surgery
Since 3D navigation has been introduced, MIS surgery has offered new opportunities for more precise and focused operations. The concept of “total navigation” using intraoperative CT navigation has changed the workflow and minimized radiation exposure in spinal surgery (43). Familiarization of these workflows with operating room staff, anesthesiologists, and surgical assistants is paramount. This is especially advantageous in cervical diseases, where anatomical landmarks are smaller compared to the other parts of the spine and reliable, accurate navigation is essential for successful operations. Our experience in navigation starting from 2D, conventional fluoroscopy to intraoperative CT-guided 3D navigation has proven to be effective and beautifully applicable in the cervical spine, and has been irreplaceable in safely performing the procedures described in this review.
Conclusions
Cervical spine surgery is indicated for those with intractable pain, neurological deficits, and/or myelopathy that are not responsive to medical therapies. Minimally invasive approaches in the cervical spine require advanced technical skills and understanding of MIS principles and limitations of each technique in order to take full advantage. The ideal procedure should improve symptoms while minimizing dissection and complications. In order to formulate the most effective surgical strategy for a particular pathology, whether MIS or open, options should be assessed on a case-by-case basis to develop an individualized plan for each patient.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jain N, Verma R, Garga UC, et al. CT and MR imaging of odontoid abnormalities: A pictorial review. Indian J Radiol Imaging 2016;26:108-19. [Crossref] [PubMed]
- Klekamp J. Chiari I malformation with and without basilar invagination: a comparative study. Neurosurg Focus 2015;38:E12. [Crossref] [PubMed]
- Kahilogullari G, Eroglu U, Yakar F, et al. Endoscopic Endonasal Approaches to Craniovertebral Junction Pathologies: A Single-Center Experience. Turk Neurosurg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Shriver MF, Kshettry VR, Sindwani R, et al. Transoral and transnasal odontoidectomy complications: A systematic review and meta-analysis. Clin Neurol Neurosurg 2016;148:121-9. [Crossref] [PubMed]
- Choi D, Crockard HA. Evolution of transoral surgery: three decades of change in patients, pathologies, and indications. Neurosurgery 2013;73:296-303; discussion 303-4. [Crossref] [PubMed]
- La Corte E, Aldana PR, Ferroli P, et al. The rhinopalatine line as a reliable predictor of the inferior extent of endonasal odontoidectomies. Neurosurg Focus 2015;38:E16. [Crossref] [PubMed]
- Goldschlager T, Hartl R, Greenfield JP, et al. The endoscopic endonasal approach to the odontoid and its impact on early extubation and feeding. J Neurosurg 2015;122:511-8. [Crossref] [PubMed]
- Hussain I, Schwartz TH, Greenfield JP. Endoscopic Endonasal Approach to the Upper Cervical Spine for Decompression of the Cervicomedullary Junction Following Occipitocervical Fusion. Clin Spine Surg 2018;31:285-92. [Crossref] [PubMed]
- Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg 2001;95:51-7. [PubMed]
- Murphey F, Simmons JC. Ruptured cervical disc. Experience with 250 cases. Am Surg 1966;32:83-8. [PubMed]
- Zeidman SM, Ducker TB. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery 1993;33:356-62. [PubMed]
- Henderson CM, Hennessy RG, Shuey HM Jr, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery 1983;13:504-12. [Crossref] [PubMed]
- Winder MJ, Thomas KC. Minimally invasive versus open approach for cervical laminoforaminotomy. Can J Neurol Sci 2011;38:262-7. [Crossref] [PubMed]
- Tumialán LM, Ponton RP, Gluf WM. Management of unilateral cervical radiculopathy in the military: the cost effectiveness of posterior cervical foraminotomy compared with anterior cervical discectomy and fusion. Neurosurg Focus 2010;28:E17. [Crossref] [PubMed]
- Foster MT, Carleton-Bland NP, Lee MK, et al. Comparison of clinical outcomes in anterior cervical discectomy versus foraminotomy for brachialgia. Br J Neurosurg 2019;33:3-7. [Crossref] [PubMed]
- Selvanathan SK, Beagrie C, Thomson S, et al. Anterior cervical discectomy and fusion versus posterior cervical foraminotomy in the treatment of brachialgia: the Leeds spinal unit experience (2008-2013). Acta Neurochir (Wien) 2015;157:1595-600. [Crossref] [PubMed]
- Dunn C, Moore J, Sahai N, et al. Minimally invasive posterior cervical foraminotomy with tubes to prevent undesired fusion: a long-term follow-up study. J Neurosurg Spine 2018;29:358-64. [Crossref] [PubMed]
- Wang TY, Lubelski D, Abdullah KG, et al. Rates of anterior cervical discectomy and fusion after initial posterior cervical foraminotomy. Spine J 2015;15:971-6. [Crossref] [PubMed]
- Skovrlj B, Gologorsky Y, Haque R, et al. Complications, outcomes, and need for fusion after minimally invasive posterior cervical foraminotomy and microdiscectomy. Spine J 2014;14:2405-11. [Crossref] [PubMed]
- Bydon M, Macki M, Kaloostian P, et al. Incidence and prognostic factors of c5 palsy: a clinical study of 1001 cases and review of the literature. Neurosurgery 2014;74:595-604; discussion 604-5. [Crossref] [PubMed]
- Zdeblick TA, Zou D, Warden KE, et al. Cervical stability after foraminotomy. A biomechanical in vitro analysis. J Bone Joint Surg Am 1992;74:22-7. [Crossref] [PubMed]
- Zdeblick TA, Abitbol JJ, Kunz DN, et al. Cervical stability after sequential capsule resection. Spine (Phila Pa 1976) 1993;18:2005-8. [Crossref] [PubMed]
- Geck MJ, Eismont FJ. Surgical options for the treatment of cervical spondylotic myelopathy. Orthop Clin North Am 2002;33:329-48. [Crossref] [PubMed]
- Bakhsheshian J, Mehta VA, Liu JC. Current Diagnosis and Management of Cervical Spondylotic Myelopathy. Global Spine J 2017;7:572-86. [Crossref] [PubMed]
- Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus 2009;27:E9. [Crossref] [PubMed]
- Spetzger U, Bertalanffy H, Naujokat C, et al. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part I: Anatomical and surgical considerations. Acta Neurochir (Wien) 1997;139:392-6. [Crossref] [PubMed]
- Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila Pa 1976) 2002;27:432-8. [Crossref] [PubMed]
- Palmer S, Turner R, Palmer R. Bilateral decompressive surgery in lumbar spinal stenosis associated with spondylolisthesis: unilateral approach and use of a microscope and tubular retractor system. Neurosurg Focus 2002;13:E4. [Crossref] [PubMed]
- Abbas SF, Spurgas MP, Szewczyk BS, et al. A comparison of minimally invasive posterior cervical decompression and open anterior cervical decompression and instrumented fusion in the surgical management of degenerative cervical myelopathy. Neurosurg Focus 2016;40:E7. [Crossref] [PubMed]
- Hur JW, Kim JS, Shin MH, et al. Minimally invasive posterior cervical decompression using tubular retractor: The technical note and early clinical outcome. Surg Neurol Int 2014;5:34. [Crossref] [PubMed]
- Wang T, Han C, Jiang H, et al. The Effect of Obesity on Clinical Outcomes After Minimally Invasive Surgery of the Spine: A Systematic Review and Meta-Analysis. World Neurosurg 2018;110:e438-49. [Crossref] [PubMed]
- Avila MJ, Walter CM, Baaj AA. Outcomes and Complications of Minimally Invasive Surgery of the Lumbar Spine in the Elderly. Cureus 2016;8:e519. [PubMed]
- Goel A. Interfacetal intra-articular spacers: Emergence of a concept. J Craniovertebr Junction Spine 2016;7:72-4. [Crossref] [PubMed]
- Goel A. Facet distraction spacers for treatment of degenerative disease of the spine: Rationale and an alternative hypothesis of spinal degeneration. J Craniovertebr Junction Spine 2010;1:65-6. [Crossref] [PubMed]
- Goel A. Facet distraction-arthrodesis technique: Can it revolutionize spinal stabilization methods? J Craniovertebr Junction Spine 2011;2:1-2. [Crossref] [PubMed]
- Goel A, Shah A, Patni N, et al. Immediate Postoperative Reversal of Disc Herniation Following Facetal Distraction-Fixation Surgery: Report of 4 Cases. World Neurosurg 2016;94:339-44. [Crossref] [PubMed]
- Siemionow K, Janusz P, Phillips FM, et al. Clinical and Radiographic Results of Indirect Decompression and Posterior Cervical Fusion for Single-Level Cervical Radiculopathy Using an Expandable Implant with 2-Year Follow-Up. J Neurol Surg A Cent Eur Neurosurg 2016;77:482-8. [Crossref] [PubMed]
- Siemionow K, Janusz P, Glowka P. Cervical cages placed bilaterally in the facet joints from a posterior approach significantly increase foraminal area. Eur Spine J 2016;25:2279-85. [Crossref] [PubMed]
- McCormack BM, Bundoc RC, Ver MR, et al. Percutaneous posterior cervical fusion with the DTRAX Facet System for single-level radiculopathy: results in 60 patients. J Neurosurg Spine 2013;18:245-54. [Crossref] [PubMed]
- Voronov LI, Siemionow KB, Havey RM, et al. Biomechanical evaluation of DTRAX((R)) posterior cervical cage stabilization with and without lateral mass fixation. Med Devices (Auckl) 2016;9:285-90. [Crossref] [PubMed]
- Gates TA, Vasudevan RR, Miller KJ, et al. A novel computer algorithm allows for volumetric and cross-sectional area analysis of indirect decompression following transpsoas lumbar arthrodesis despite variations in MRI technique. J Clin Neurosci 2014;21:499-502. [Crossref] [PubMed]
- Siemionow KB, Glowka P, Blok RJ, et al. Perioperative complications in patients treated with posterior cervical fusion and bilateral cages. J Craniovertebr Junction Spine 2017;8:342-9. [Crossref] [PubMed]
- Navarro-Ramirez R, Lang G, Lian X, et al. Total Navigation in Spine Surgery; A Concise Guide to Eliminate Fluoroscopy Using a Portable Intraoperative Computed Tomography 3-Dimensional Navigation System. World Neurosurg 2017;100:325-35. [Crossref] [PubMed]


