Abstract
Background Veterans RAND 12-item (VR-12) physical component score (PCS) has been validated in both veteran and US citizen populations; however, its use for spine surgery populations has not been evaluated. This study aims to correlate the VR-12 PCS survey with legacy patient-reported outcome measures (PROMs) in patients undergoing minimally invasive transforaminal lumbar interbody fusion (MIS TLIF).
Methods A prospective surgical database was retrospectively assessed for MIS TLIFs performed at 1 level from March 2015 to June 2019. Inclusion criteria were elective procedures for degenerative spinal pathology. Patients were excluded if they had surgery for metastatic, traumatic, or infectious etiologies or had incomplete preoperative 12-item Short Form (SF-12) PCS or Patient-Reported Outcomes Measurement Information System physical function (PROMIS-PF) survey. Additionally, patients with any incomplete VR-12 PCS surveys through 1 year were excluded. Demographics and perioperative characteristics were recorded. Mean postoperative PROM scores and score difference from preoperative baseline were calculated at each postoperative timepoint through 1 year. The relationship of VR-12-PCS with SF-12-PCS and PROMIS PF was evaluated with a Pearson’s correlation coefficient and time-independent partial correlation.
Results A total of 59 patients underwent single-level MIS TLIFs. The cohort was 44.1% women with an average age of 53.8 years, and 52.5% were obese (body mass index ≥30 kg/m2). The VR-12 PCS, SF-12 PCS, and PROMIS PF surveys had significant improvements from baseline to the 6 month through 1 year postoperative timepoints (P ≤ 0.001, all). All timepoints revealed strong VR-12-PCS correlations with SF-12-PCS and PROMIS PF (all P ≤ 0.001).
Conclusion VR-12 PCS, SF-12 PCS, and PROMIS PF scores all indicate statistically significant improvements in physical function for patients following MIS TLIF. VR-12 PCS was strongly correlated with the historically validated SF-12 PCS system as well as with the more recent PROMIS PF survey. Our observations give weight to utilizing the VR-12 PCS survey as a valid measure of physical function among patients undergoing MIS TLIF.
Clinical Relevance This study validates VR-12 PCS to measure physical function for TLIF patients.
Level of Evidence 4.
- lumbar surgery
- patient-reported outcomes
- minimally invasive
- low back pain
- outcomes
- Oswestry Disability Index (ODI)
- radiculopathy
- stenosis
- disability
- survey
- questionnaire
INTRODUCTION
The United States spends more on health care annually than any country in the world, and many scholars are concerned that a considerable portion is wasteful.1,2 Thus, there has been a growing emphasis on value-based and cost-effective care. This value is often quantified and presented in terms of patient-reported outcome measures (PROMs). These measures have been shown to not only correlate with patient satisfaction,3,4 but also help predict perioperative outcomes.5,6 PROMs have also begun to influence both provider and hospital reimbursement.7 Therefore, understanding which of these measures are valid and clinically relevant is paramount.
There have been a number of disease-specific physical outcome measures developed for lumbar spine pathology, including the Zurich Claudication Questionnaire and the Oswestry Disability Index (ODI).8,9 Despite the initial success and utility of these measures, there has been a push to utilize metrics that are universal across chronic conditions and demographic groups. The use of such measures can help to better compare the disability of very different conditions (eg, knee osteoarthritis vs heart failure), thereby guiding allocation of resources and reimbursement. In response, the RAND Corporation developed the 36-Item Short Form Survey (SF-36) and the abbreviated 12-Item Survey (SF-12) for widespread use across a number of specialties.10,11 Since its introduction, the Short Form Survey has not only been the most frequently utilized health-related quality-of-life assessment in spine surgery, but it has also been recognized by the Food and Drug Administration in its recommendations for Spine Investigational Device Exemption.11
The Veterans RAND 12-Item Survey (VR-12) is a modified version of the Short Form Survey that aims to increase the precision of the instrument and has been administered and validated through more than 7 million questionnaires nationwide.12,13 The VR-12 is a valuable metric for several reasons. It is included in the annual Ambulatory Care Survey of Health Care Experience of Patients13 and the Health Outcomes Survey,14 both of which are widely administered samplings that help guide reimbursement and safety/performance rankings. Scores from the VR-12 can be converted into the newly developed Patient-Reported Outcomes Measurement Information System (PROMIS) through the PROsetta Stone initiative.15,16 The Centers for Medicare & Medicaid Services has officially recommended the use of PRO measures such as the VR-12 that are validated, nonproprietary, and short in duration.17 Finally, the VR-12 in particular has been identified by the Centers for Medicare & Medicaid Services as a preferred outcome measure in the field of orthopedic surgery and is now an official metric in the Comprehensive Care for Joint Replacement bundled payment model.17,18 Continued validation efforts of the VR-12 in orthopedic and spine surgery are therefore critical.
Low back pain and degenerative pathology of the lumbar spine are leading causes of disability and rising health care costs.19 Instrumented transforaminal lumbar interbody fusion (TLIF) is a popular surgical procedure that is used to treat a number of these degenerative pathologies, including degenerative disc disease, spondylolisthesis, and recurrent disc herniation.20 The use of minimally invasive spinal (MIS) techniques with the TLIF procedure has been associated with decreased blood loss, shorter length of hospital stay, decreased narcotic usage, decreased infection rate, and greater cost-effectiveness when compared with the open procedure.21–24 To truly quantify the value of the procedure, high-quality validated patient-reported outcome metrics are needed. Despite the use of the VR-12 in prior research, the outcome measure has yet to be validated in this patient population.25–27 Thus, the goal of the present study was to assess the validity of the VR-12 physical component score (PCS) in relation to other physical outcome metrics previously validated for lumbar spine pathology, for patients undergoing MIS TLIF surgery.
METHODS
Study Population
Following institutional review board approval (ORA #14051301) and obtaining consent, we retrospectively reviewed a prospectively recorded surgical database for patients who underwent single-level MIS TLIFs from March 2015 to June 2019. Patients were included if they underwent an elective procedure for degenerative spinal pathology. Exclusion criteria were procedures for metastatic disease, trauma, or infections, along with missing preoperative surveys for SF-12 or PROMIS physical function (PF). Additionally, patients with any incomplete VR-12 PCS surveys from the preoperative through 1-year postoperative timepoint were excluded from the study.
Data Collection
Patient demographics of age, tobacco use, sex, body mass index (by either <30 or ≥30 kg/m2), comorbidity burden as evaluated by the Charlson Comorbidity Index, and baseline spinal pathologies were recorded. Perioperative characteristics, including operative duration (from skin incision to closure), estimated blood loss, and duration of hospital stay, were also recorded. Spinal pathologies were classified as spondylolisthesis, herniated nucleus pulposus, degenerative disc disease, central/spinal stenosis, or foraminal stenosis. Follow-up rates at all timepoints were calculated based on completion of postoperative VR-12 PCS.
Statistical Analysis
All analysis was completed utilizing Stata SE 16.1 (College Station, Texas, USA). Descriptive statistics including mean and SD values were reported for baseline characteristics. Mean postoperative VR-12 PCS, SF-12 PCS, and PROMIS-PF scores were calculated at both pre- and postoperative timepoints (eg, 6 weeks, 12 weeks, 6 months, and 1 year). A mean score difference was calculated to evaluate the change from baseline preoperative scores to each postoperative timepoint for all evaluated PROMs. Scatterplots were constructed to visualize the association of VR-12 PCS with SF-12 PCS and PROMIS-PF at each timepoint. We evaluated the relationship of VR-12 PCS with SF-12 PCS and PROMIS PF scores with a Pearson’s correlation coefficient and time-independent partial correlation. Correlation strength was assessed by the following categories: 0.1 ≤ |r| < 0.3 = low; 0.3 ≤ |r| < 0.5 = moderate; |r| ≥0.5 = strong. Significance was set at an alpha = 0.05.
RESULTS
Population Characteristics
Our study consisted of 59 patients who underwent single-level MIS TLIFs. The majority were men (55.9%), nonsmokers (91.5%), obese (52.5%, body mass index ≥30 kg/m2), and a mean age of 53.8 years (Table 1). The 3 most common preoperative spinal pathologies were central stenosis (88.1%), degenerative spondylolisthesis (63.3%), and foraminal stenosis (50.9%).
Baseline characteristics of study population.
Outcomes
The average procedure duration was 121.1 minutes with an estimated blood loss of 56.2 mL. The mean length of stay in the hospital following surgery was 34.8 hours. Follow-up rates were 100.0% at all postoperative timepoints. The VR-12 PCS, SF-12 PCS, and PROMIS-PF metrics demonstrated statistically significant improvements compared with preoperative values at the 12-week postoperative timepoint through 1 year (all P < 0.001, Table 2). Preoperative scores were compared with all evaluated postoperative timepoints with paired t tests (P ≤ 0.001 for 6 weeks, 12 weeks, 6 months, and 1 year).
Postoperative changes in physical function scores.
Validation
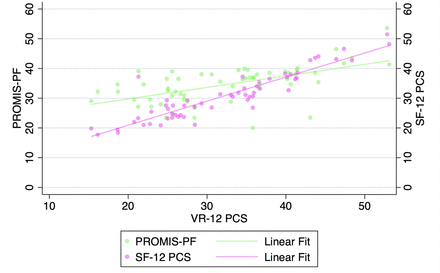
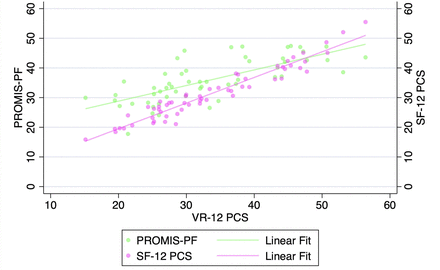
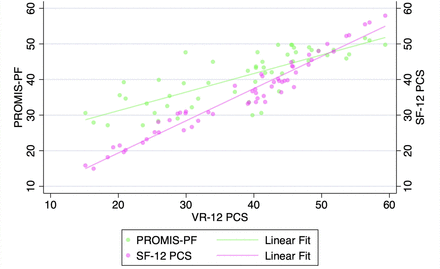
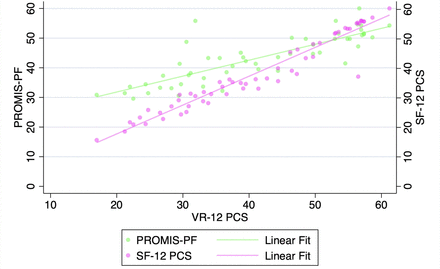
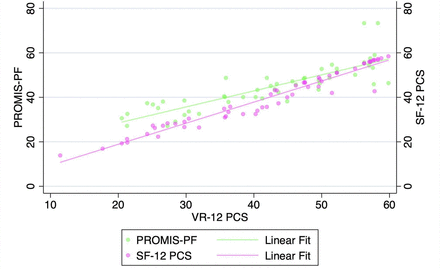
VR-12 PCS demonstrated a strong correlation with SF-12 PCS and PROMIS-PF at all pre- and postoperative timepoints when assessed with Pearson’s correlation coefficients and with partial correlation time-controlled coefficients (all P < 0.001, Table 3, Figures 1–5). At all timepoints, VR-12 PCS revealed a stronger correlation with SF-12 PCS (|r| ≥ 0.913, all) compared with PROMIS-PF (|r| ≥ 0.572 and |r| ≤0.845, all) (Table 3).
Association of VR-12 with SF-12 and PROMIS-PF following minimally invasive surgery transforaminal lumbar interbody fusion.
Correlation of Veterans RAND 12-item physical component score (VR-12 PCS) with patient-reported outcomes measurement information system physical function (PROMIS-PF) and 12-item Short Form physical component score (SF-12 PCS) at the preoperative timepoint.
Correlation of Veterans RAND 12-item physical component score (VR-12 PCS) with patient-reported outcomes measurement information system physical function (PROMIS-PF) and 12-item Short Form physical component score (SF-12 PCS) at the 6-wk postoperative timepoint.
Correlation of Veterans RAND 12-item physical component score (VR-12 PCS) with patient-reported outcomes measurement information system physical function (PROMIS-PF) and 12-item Short Form physical component score (SF-12 PCS) at the 12-wk postoperative timepoint.
Correlation of Veterans RAND 12-item physical component score (VR-12 PCS) with patient-reported outcomes measurement information system physical function (PROMIS-PF) and 12-item Short Form physical component score (SF-12 PCS) at the 6-mo postoperative timepoint.
Correlation of Veterans RAND 12-item physical component score (VR-12 PCS) with patient-reported outcomes measurement information system physical function (PROMIS-PF) and 12-item Short Form physical component score (SF-12 PCS) at the 1-y postoperative timepoint.
DISCUSSION
The use of high-quality validated outcome metrics in spine surgery is critical. There has been a growing demand for measures that are inexpensive or freely available, applicable across a number of specialties, and easy to complete. As a result, use of metrics such as the VR-12 PCS has risen considerably in spine surgery,28–30 other areas of orthopedic surgery,31,32 and nonorthopedic elective surgeries.33 However, the VR-12 PCS metric had yet to be formally validated among patients with degenerative pathology of the lumbar spine.
In the present study, we compared VR-12 PCS scores with SF-12 PCS and PROMIS-PF scores in patients undergoing MIS TLIF. The patients in our sample were generally young and healthy, with a mean age of 53.8 years and mean Charlson Comorbidity Index of 1.8. The most common spinal pathologies were central stenosis, degenerative spondylolisthesis, and foraminal stenosis. Patients generally did well following surgery, reporting statistically significant improvements in each of the 3 physical outcome measures at 12 weeks, 6 months, and at 1 year postoperatively. The VR-12 PCS exhibited strong correlation coefficients with both the SF-12 PCS and PROMIS-PF at all timepoints. As a result, our findings are strongly suggestive of VR-12 PCS score criterion validity in patients undergoing MIS TLIF for degenerative lumbar pathology.
Confidence in the validity of the VR-12 PCS is dependent on the validity of the metrics to which it has been previously compared (ie, SF-12 PCS and PROMIS-PF). As previously mentioned, both the SF-12 and SF-36 surveys along with PROMIS-PF have been validated in patient populations with degenerative conditions of the lumbar spine.34,35 Guilfoyle et al analyzed 203 patients with lumbar disc herniations and 177 patients with lumbar stenosis and compared scores from the then-novel SF-36 with the long-time standards of the Roland-Morris Disability Index, the Hospital Anxiety and Depression Scale, and the visual analog scale.34 The 3 domains of the SF-36 (physical function, bodily pain, and mental health) each exhibited statistically significant correlation to the standard measures for these patients. Similarly, following the release of PROMIS, Patel et al assessed 98 consecutive patients undergoing lumbar decompression for stenosis with a number of PROMs.35 The established legacy measures included the ODI, Zurich Claudication Questionnaire, and the SF-12. The team found that PROMIS scores (physical function, pain interference, and pain behavior) correlated to the legacy measures with moderate to high strength, demonstrating both convergent validity and known groups validity based on their statistical analysis.
To the authors’ knowledge, there have been no prior attempts to validate the VR-12 PCS in patients with degenerative lumbar pathology undergoing MIS TLIF. However, the findings of the present study are consistent with previous research utilizing the VR-12 PCS as a spine surgery perioperative outcome measure. Khan et al analyzed 100 consecutive patients with lumbar disc herniation who underwent discectomy surgery utilizing a number of PROMs.28 They found that patients reported an improvement in mean VR-12 PCS (−9.79 points) in addition to SF-12 PCS (3.22 points) and ODI (31.33 points). Gornet et al performed an analysis of 1968 patients undergoing spine surgery (1559 lumbar and 409 cervical) who had completed pre- and postoperative SF-36 surveys.36 The team was able to collect VR-12 data (PCS and mental component score) based on the responses to the SF-36. They found that VR-12 (PCS and mental component score) detected clinical improvement for the vast majority of patients, with a mean improvement of 10.13 points for the lumbar spine surgery group. Both groups found the VR-12 questionnaire easy to complete and interpret relative to the other utilized outcome measures.
Although there is an impetus to use the National Institutes of Health-funded PROMIS-PF, the VR-12 PCS remains an important outcome measure with unique advantages. PROMIS often utilizes computer adaptive testing to measure health outcomes. This computer adaptive testing technology employs an algorithmically based question system such that the outcome measure scores can be determined with high specificity and fewer questions relative to comparable metrics. As a result, however, PROMIS administration requires a computer or other electronic device. The VR-12 on the other hand, can be administered via traditional pencil and paper, a modality that remains common for survey recruitment and administration.37 For practices lacking the capital to invest in electronic devices, this VR-12 is an acceptable alternative. Furthermore, Patel et al found that completion time for the SF-12 (comparable in duration to the VR-12) was not substantially longer than completion time for PROMIS (3.0 vs 2.6 minutes, respectively). Finally, if a practice or group does fully adopt the PROMIS system at a later date, the historical VR-12 PCS data can be converted into PROMIS-PF format.16,38
The present study has several limitations. Physical function scores were assessed and analyzed with a final timepoint of 1 year postoperative. This duration compares favorably with previously discussed validation efforts by Patel et al35 and Guilfoyle et al,34 both of whom used a 3-month minimum follow-up for inclusion. However, it has been suggested that patients undergoing lumbar fusion should be followed clinically for at least 2 years in order to effectively detect achievement of the minimal clinically important difference in disability outcome measures.39 Although the goal of the present study was not to assess the achievement of the minimal clinically important difference following MIS TLIF, it is likely that this patient population would continue to experience improvement in outcome measures such as the VR-12 PCS through the 2-year postoperative mark. Although the VR-12 PCS exhibited strong correlation with both the SF-12 PCS and the PROMIS-PF at all timepoints prior, further research is needed to determine the strength of this correlation at longer-term follow-up.
CONCLUSIONS
In this study, we found that VR-12 PCS is a valid outcome measure that correlates strongly with other metrics validated for lumbar spine pathology. For patients undergoing MIS TLIF surgery, the VR-12 PCS offers a fast, easy to complete, nonproprietary, and universally applicable physical outcome assessment. The VR-12 PCS can now also be converted into the PROMIS PF framework. Given the multitude of outcome measures available and the concern for survey fatigue, the VR-12 PCS appears to be a strong and comprehensive physical outcome assessment for patients undergoing MIS TLIF. Clinicians should feel comfortable using this tool as their physical outcome measure for patients undergoing this procedure.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
Disclosure The authors report no financial disclosures related to this article.
IRB Approval ORA #14051301.
Disclosures Kern Singh discloses that he has received grants or contracts from the Cervical Spine Research Society; royalties or licenses from RTI Surgical, Zimmer Biomet, Stryker, Lippincott Williams & Wilkins, Theime, Jaypee Publishing, and Slack Publishing; consulting fees from K2M and Zimmer Biomet; patents planned, issued, or pending with TDi LLC; and leadership or fiduciary role on Vitals 5 LLC, TDi LLC, Minimally Invasive Spine Study Group, Contemporary Spine Surgery, Orthopedics Today, and Vertebral Columns. The remaining authors have no disclosures.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2022 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.