ABSTRACT
Background: Paragangliomas (PGLs) are rare neuroendocrine tumors that can arise from any autonomic ganglion of the body. Most PGLs do not metastasize. Here, we present a rare case of metastatic PGL of the spine in a patient with a germline pathogenic succinate dehydrogenase subunit B (SDHB) mutation.
Methods: In addition to a case report we provide a literature review of metastatic spinal PGL to highlight the importance of genetic testing and long-term surveillance of these patients.
Results: A 45-year-old woman with history of spinal nerve root PGL, 17 years prior, presented with back pain of several months' duration. Imaging revealed multilevel lytic lesions throughout the cervical, thoracic, and lumbar spine as well as involvement of the right mandibular condyle and clavicle. Percutaneous biopsy of the L1 spinal lesion confirmed metastatic PGL and the patient underwent posterior tumor resection and instrumented fusion of T7–T11. Postoperatively the patient was found to have a pathogenic SDHB deletion.
Conclusions: Patients with SDHx mutation, particularly SDHB, have increased risk of developing metastatic PGLs. Consequently, these individuals require long-term surveillance given the risk for developing new tumors or disease recurrence, even years to decades after primary tumor resection. Surgical management of spinal metastatic PGL involves correcting spinal instability, minimizing tumor burden, and alleviating epidural cord compression. In patients with metastatic PGL of the spine, genetic testing should be considered.
INTRODUCTION
Paragangliomas (PGLs) are rare neuroendocrine tumors that can develop anywhere along the sympathetic and parasympathetic ganglia of the body with an estimated prevalence of 0.2 to 1 per 100,000.1,2 When PGLs arise from chromaffin cells they frequently overproduce catecholamines. PGLs arising from adrenal chromaffin cells are commonly known as pheochromocytomas and account for 80%–85% of chromaffin cell PGLs.3 Extra-adrenal PGLs are less frequent, are primarily found along the parasympathetic ganglia of the head and neck, and are more likely to be biochemically silent.4,5 Extra-adrenal PGLs generally present between ages 40 and 50 with symptoms of mass effect specific to their location of origin.5–7
Most PGLs do not metastasize, with only 10%–17% ultimately being metastatic, although extra-adrenal PGLs are thought to have greater metastatic potential.8,9 Diagnosis of metastatic PGL is difficult as there are no reliable cellular or molecular markers of metastatic disease, and thus progression is necessary for diagnosis.8,10 Given the rarity of metastatic disease, there is a relative paucity of literature on metastatic extra-adrenal PGL, especially for spinal metastasis.4,10–14 Here, we present a report of a patient with metastatic extra-adrenal PGL of the spine presenting 27 years after resection of primary spinal nerve root PGL who was found to have a germline succinate dehydrogenase subunit B (SDHB) mutation.
CASE PRESENTATION
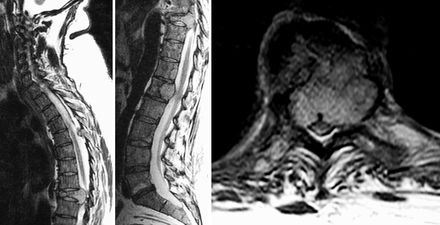
A 45-year-old woman with history of a large (> 8 cm) spinal nerve root PGL, status postresection 27 years prior at an outside hospital, with a recurrence in the right tibia, status postresection 6 years prior also at an outside hospital, presented for care at our institution with severe thoracic back pain radiating around the rib cage for several months' duration, which was worse with movement. She endorsed feeling weak, although she was able to ambulate normally. She denied lower extremity symptoms, saddle anesthesia, and changes in urination or defecation. Physical exam was normal except for tenderness to palpation along the lower thoracic spine. She reported no overt signs of catecholamine excess such as tremors, headaches, visual symptoms, palpitations, weight loss, and diaphoresis. Preoperatively, both her blood pressure and pulse were within normal limits and she was not on any antihypertensive medications. On imaging with computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography, she was found to have multilevel lytic lesions throughout the cervical, thoracic, and lumbar spine at C1, C4–C7, L1–L3, as well as involvement of the right mandibular condyle and clavicle. She had compression fractures of the C3 and T9 vertebral bodies both with greater than 80% loss of height, and retropulsion causing moderate-to-severe spinal canal stenosis. Spinal canal extension was present at T4, T5, T9, L1, and L4, and most severe at T9, which was considered to be the symptomatic level (Figure 1).
Preoperative T2 magnetic resonance imaging (MRI) complete spine images showing extensive metastatic disease of the spine. (Left) Sagittal T2 MRI of the upper spine showing pathologic compression fracture of the T9 vertebral body with retropulsion of the posterior vertebral body obliterating the cerebrospinal fluid space. (Middle) Sagittal T2 MRI of the lower spine showing extensive metastatic disease throughout the lumbar spine. (Right) Axial T2 MRI at the level of the T9 vertebral body.
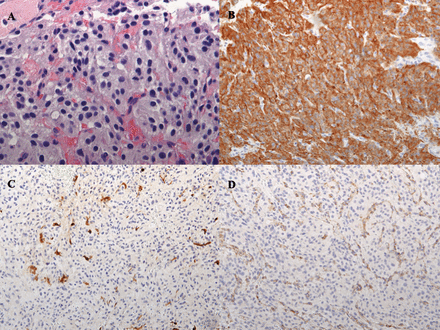
On the day after admission, percutaneous biopsy was performed of the L1 soft tissue lesion, and this confirmed metastatic PGL. Histologic sections of the biopsy specimen contained abundant groups of nested cells characterized by mild anisonucleosis, stippled nuclear chromatin, and abundant pale eosinophilic and slightly granular cytoplasm. Mitotic figures were seen at 7 in 10 high-power fields (Figure 2A). Immunohistochemical stains for S100, synaptophysin, chromogranin, and succinate dehydrogenase subunit B (SDHB) were performed. Tumor cells stained positive for synaptophysin (Figure 2B) and chromogranin. The S100 stain highlighted a fragmented network of sustenacular cells surrounding the neoplastic cells (Figure 2C). SDHB showed intracytoplasmic granular positivity within vascular endothelium but complete loss of staining in the tumor cells (Figure 2D), indicative of an underlying SDHx gene mutation.
Histologic and immunohistochemical sections of metastatic paraganglioma biopsy specimen. (A) Hematoxylin-eosin stain of metastatic paraganglioma (×60). Histologic sections of the biopsy specimen showing an epithelioid proliferation of cells with nested architecture (“Zellballen”) with a delicate tumor vascular network between nests. The epithelioid cells show round to ovoid nuclei, minimal nuclear pleomorphism, and stippled chromatin with abundant granular amphophilic cytoplasm. Mitotic figures are seen at 7 in 10 high-power fields. (B) Immunohistochemical stain for synaptophysin showing positive granular cytoplasmic staining in tumor cells (×20). (C) Immunohistochemical stain for S-100 highlighting fragmented network of sustenacular cells surrounding tumor cells (×20). (D) Immunohistochemical stain for succinate dehydrogenase subunit B (SDHB) showing absence of SDHB among tumor cells, while maintaining positive internal control within vascular endothelium (×20).
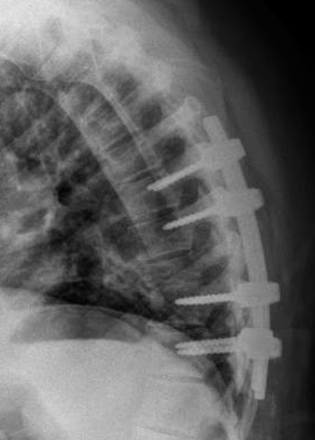
Given clinical concern for spinal instability and epidural cord compression, the patient underwent lateral extracavitary approach for partial corpectomy and laminectomy for tumor resection at T9 and posterior instrumented fusion of T7–T11. The patient tolerated the procedure well with 400 mL of blood loss and no hypertensive complications. In consultation with anesthesiology, preoperative alpha blockade was not performed.15 Grossly, the tumor appeared as fragments of red-brown soft tumor mixed with blood and trabecular bone fragments. Histologic and immunohistochemical analysis was consistent with recent biopsy. A postoperative x-ray was obtained to confirm adequate positioning of hardware (Figure 3). Patient was discharged on postoperative day 5 with a thoracic lumbosacral orthosis brace and cervical collar. Although no family history of pheochromocytoma or PGL was obtained on review, germline testing was recommended with particular concern for SDHB mutation given negative SDHB immunohistochemistry and the aggressive metastatic nature of the PGL. The patient was found to have a pathogenic SDHB deletion (c.166_170delCCTCA) which resulted in a frame shift and protein truncation (p.P56Yfs*5). Genetic testing was arranged for at-risk relatives, where several affected individuals were identified.
Postoperative sagittal x-ray of the spine showing instrumentation.
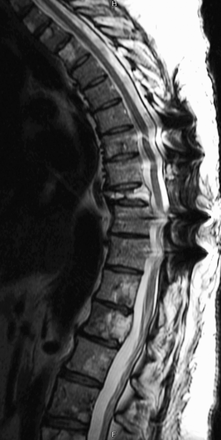
At 18 months after surgery the patient's metastatic disease is stable (Figure 4). She has received 10 sessions of radiation to the spine and 8 cycles of chemotherapy with cyclophosphamide, dacarbazine, and vincristine. Additionally, she has been receiving monthly octreotide and denosumab injections to promote disease stabilization and prevent further bone loss, respectively. Given the patient's advanced disease, additional screening beyond standard response surveillance was not pursued.
Post-operative T2 magnetic resonance imaging of thoracic spine at 1.5 years showing stable disease.
RESULTS AND DISCUSSION
Metastatic extra-adrenal PGL to the spine is a rare phenomenon with limited case reports and 2 small case series described in the English literature (Table 1).4,10,11 Here, we present what is, to our knowledge, the fourth case report of metastatic PGL to the spine found to have a mutation of an SDH subunit, (SDHA, SDHB, SDHC, SDHD) or assembly factor (SDH-AF2).4 While there are limited data available on the metastatic tendencies of extra-adrenal PGL, studies looking at both metastatic pheochromocytomas and extra-adrenal PGLs have found bone to be the most common site of metastasis followed by liver and lung.16–19 The spine is the most common site of bone metastasis.16 In this case our patient had an interval of 27 years between total resection of her primary tumor and spinal metastasis. This extended interval is consistent with previous case reports as shown in Table 1.10,12,20
Previous reports of metastatic extra-adrenal PGL to the spine.
Genetic testing is recommended for all patients with PGLs and their first-degree relatives.3,43 Over the past several years it has been shown that 20%–40% of patients with pheochromocytoma and extra-adrenal PGL have a germline mutation in SDHx, NF1, VHL, or RET.44–46 While mutations in NF1, VHL, and RET genes cause well-characterized hereditary syndromes, the association between SDHx mutations and PGLs was more recently elucidated.6,47–49 SDH is a mitochondrial enzyme complex that plays a role in both the tricarboxylic acid cycle and in the electron transport chain. SDH genes function as classical tumor suppressor genes where somatic loss of heterozygosity of the wild-type allele is observed in tumors.6,50 While the precise pathogenic mechanism of SDH-mutation-dependent tumor formation is incompletely understood, the leading theory revolves around the role of succinate as an oncometabolite.51 Specifically, it is thought that disruption of SDH complex function, which leads to an accumulation of succinate, increases the risk of cancer because succinate accumulation competitively inhibits α-ketoglutarate dependent enzymes such as prolyl hydroxylases and histone demethylases. Inhibition of hypoxia-inducible factor prolyl hydroxylases leads to the stabilization of hypoxia-inducible factors, which normally promote angiogenesis and cell survival in hypoxic conditions. Inhibition of histone demethylases causes a cell to adopt a hypermethylator phenotype that is thought to silence genes associated with neuroendocrine differentiation.52–54 SDHx mutations are the most frequent hereditary cause of extra-adrenal PGLs with autosomal-dominant mutations in SDHD and SDHB being the most common.6,49 Immunohistochemical and genetic testing all PGLs for SDHx mutation, and in particular for SDHB mutation, is critically important as 30%–70% of metastatic PGLs have been found to have an SDHB germline mutation.48,55,56 Thus, while there are no definite histological markers of metastatic PGL, SDHB mutation is a strong independent predictor in addition to primary tumor size > 5 cm and extra-adrenal location.8 Interestingly, although our patient's primary tumor was reportedly > 8 cm in size and extra-adrenal in location she was not screened for SDHB mutation prior to receiving care at our institution for her spinal metastasis.
Whole-body scanning for detection of metastatic PGL is recommended at the time of primary tumor detection.3 Metastatic disease can be detected through a combination of anatomical (CT, MRI) and scintigraphic imaging techniques (fluorodeoxyglucose positron emission tomography, metaiodobenzylguanidine scintigraphy, and more recently somatostatin receptor scintigraphy with 68Ga-DOTATATE).57,58 Fluorodeoxyglucose positron emission tomography is currently the preferred imaging technique for detecting metastatic disease although several recent studies suggest that 68Ga-DOTATATE may have greater sensitivity particularly in the context of SDH-related disease.3,59,60 Long-term imaging follow-up in addition to annual clinical evaluation and laboratory testing is required for all patients with PGLs as approximately 50% of metastatic PGLs present metachronously and in particular for patients with SDHx mutations, who have an increased risk for metastatic disease.19 At this time no clear guidelines exist regarding the optimal frequency of imaging for PGL patients.3 In terms of laboratory testing, the Endocrine Society recommends lifelong annual testing of plasma or urine metanephrine levels to assess for recurrent or persistent disease.3
Treatment of metastatic PGL of the spine involves a combination of surgery, radiation therapy, and chemotherapy. As with other metastatic tumors of the spine the primary goals of surgery are management of spinal instability caused by lytic lesions, and decompression of the spinal cord secondary to any epidural tumor.61 Surgical resection of the primary tumor has been shown to improve overall survival in cases of synchronous metastatic PGL.62 Radiation therapy is the primary of method of local control for metastatic disease that is unresectable. Chemotherapy is reserved for widely metastatic disease with cyclophosphamide, dacarbazine, and vincristine being the preferred regimen.63 Overall, metastatic PGL is difficult to treat with 5-year overall survival at approximately 60%.61
In conclusion, metastasis to the spine is a rare but important complication in patients with PGL that can lead to significant pain and disability. Genetic testing is recommended for all patients with PGLs. Patients with SDHx mutations are more likely to develop metastatic disease and SDHx mutation status is the current best predictor of metastatic PGL. SDHx mutation carriers must have frequent long-term imaging surveillance performed given the potential for metastasis several years to decades after primary resection. Neurosurgical management of metastatic PGL of the spine involves correcting spinal instability and alleviating epidural cord compression. When caring for patient with metastatic PGL of the spine, genetic testing for patients and their families should be considered.
Footnotes
Disclosures and COI: None declared. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patient's written informed consent for publication was obtained.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2020 ISASS