Abstract
Background Chronic low back pain secondary to degenerative disc disease is a significant public health issue worldwide, contributing to substantial health care burdens and patient disability. Anterior lumbar interbody fusion (ALIF) has emerged as a promising surgical solution, offering benefits such as disc height restoration, reduced neural compression, and improved spinal alignment. This study evaluates the efficacy of stand-alone ALIF using polyetheretherketone (PEEK) cages, structural femoral head allografts, and recombinant human bone morphogenetic protein-2 (rhBMP-2) in treating discogenic low back pain caused by degenerative disc disease.
Methods This prospective case series study included 1335 patients who underwent stand-alone ALIF by a single surgeon. The surgical construct involved PEEK cages with structural femoral allograft dowels and rhBMP-2, supplemented by anterior fixation. Patient-reported outcome measures, including the visual analog scale for back and leg pain, the Oswestry Disability Index, the Roland-Morris Disability Questionnaire, and patient satisfaction, were monitored over 12 months.
Results The overall fusion rate was 99.6%, with pseudoarthrosis occurring in 0.2% of patients. Lower fusion rates were observed in patients older than 65 years and those using the Brantigan cage. Significant improvements were seen in visual analog scale for back and leg pain, Oswestry Disability Index, and Roland-Morris Disability Questionnaire scores from baseline, with most scores exceeding the substantial clinical benefit thresholds. More than 85% of patients reported “Excellent” or “Good” outcomes.
Conclusions Stand-alone ALIF, augmented with rhBMP-2 and structural femoral head allografts, can enhance mechanical stability, fusion rates, and radiographic assessment. This integrated approach achieves successful spinal fusion and positive clinical outcomes for patients with refractory discogenic low back pain.
Clinical Relevance Stand-alone ALIF with PEEK cages, structural femoral head allografts, and rhBMP-2 demonstrates high fusion rates and significant clinical improvements in patients with discogenic low back pain. This approach enhances spinal stability and promotes biological healing, making it a reliable and effective surgical option.
Level of Evidence 4.
Introduction
Chronic low back pain (CLBP) remains a significant local and global health issue, profoundly impacting individuals and health care systems worldwide.1 The Australian Burden of Disease study highlights back pain as a leading cause of nonfatal burden.2 With an estimated 3.7 million Australians suffering from chronic back problems, the economic toll exceeds AU$4.8 billion annually, and this figure is expected to rise.2 Such data underscore the importance of finding practical solutions for CLBP.3
Within the spectrum of CLBP, discogenic origins contribute to neurogenic, biomechanical, and biochemical changes within the intervertebral disc.4 Progressive degeneration results in loss of disc height and associated neural compression, potentially leading to debilitating radiculopathy.5 Despite the proliferation of nonsurgical interventions, their efficacy remains contentious, prompting the exploration of surgical options for refractory cases.6–9
Conventional spinal fusion is a viable option when conservative measures prove inadequate. However, debates persist regarding the clinical efficacy of fusion procedures, highlighting the need for a nuanced understanding and optimization of fusion techniques.10,11 Reliable bony fusion, governed by intricate physiological and biomechanical principles, is central to this effort.12,13
The contemporary landscape of spinal fusion is witnessing a surge in innovation, particularly with interbody fusion (IBF) cages. Polyetheretherketone (PEEK), known for its biomechanical compatibility with human bone, has emerged as a preferred biomaterial for IBF. Despite its mechanical advantages, concerns regarding osseointegration have led to advancements in surface modifications to enhance bone-implant interactions.14–16
Amid these advancements, anterior lumbar interbody fusion (ALIF) is a technique that restores disc height and lordosis, reducing back and leg symptoms.17 Moreover, it plays a crucial role in protecting the dynamic stabilizers and the erector spinae muscles. By combining meticulous surgical technique with Giannoudis’s Diamond Concept principles,18 ALIF with PEEK cages, supplementary fixation, structural femoral head allograft, and recombinant human bone morphogenetic protein-2 (rhBMP-2) offers an integrated approach to successful fusion.19–23 This approach sets a paradigm for addressing the complexities of CLBP with precision and efficacy.24 PEEK cages provide essential structural support, BMP-2 stimulates bone growth, and allografts scaffold new bone development.25,26
The present study evaluates the effectiveness of standalone ALIF in managing discogenic low back pain. The study focuses on the benefits of this approach, particularly its impact on spinal stability, biological healing, pain relief, and functional improvement. It posits that integrating standalone ALIF with PEEK cages, structural femoral head allografts, and rhBMP-2 enhances spinal stability and promotes reliable, consistent biological healing.
Methods
This prospective study involved 1335 patients who underwent anterior lumbar reconstruction surgery performed by a single surgeon at a single institution with a follow-up period of 12 months. The ALIF surgical construct utilized PEEK cages with structural femoral allograft dowels and rhBMP-2, supplemented by anterior fixation (either a plate-screw construct or integrated screws). Surgical access was gained through a midline rectus split, followed by a left or right retroperitoneal approach. When approaching higher levels, a left retroperitoneal approach was used.
Critical outcomes assessed were ALIF fusion rates and patient-reported outcome measures (PROMs). Pain was measured using the visual analog scale for the back (VAS-B) and leg (VAS-L). Disability was assessed using the Oswestry Disability Index (ODI) and the Roland-Morris Disability Questionnaire (RMDQ). Patient satisfaction was evaluated using a 4-point scale: poor, satisfactory, good, and excellent.27,28
Fusion outcomes were categorized into 4 groups: first, “fused (CT + XR),” confirmed by fine-cut computed tomography (CT) and erect flexion-extension radiography; second, “fused (XR),” confirmed by erect flexion-extension radiography only; third, “pseudoarthrosis,” indicating no fusion; and finally, “unavailable.” The change in imaging modality throughout this study—from dynamic radiography alone to the inclusion of fine-cut CT—reflects evolving recommendations in spinal literature regarding the gold standard for fusion assessment.29 The criteria for fusion focused on the presence of a continuous bony bridge across the intervertebral space, ensuring no gaps or lucency at the bone-graft interface (Figure 1). Additionally, the absence of motion on flexion-extension x-ray images and the integrity of surgical hardware, such as no signs of screw loosening or plate migration, were evaluated to confirm successful fusion.30
Dynamic x-ray images showing stability across the anterior lumbar interbody fusion construct in flexion (A) and extension (B). Fine-cut computed tomography images showing confluent bridging bone assessed on sagittal (C) and coronal (D) sequences.
Subgroup analyses were conducted based on several factors, including ALIF levels, age groups, review types, cage types, and ALIF groups (Figure 2). Patients were divided into 2 ALIF groups based on surgical levels: “higher” for L2-3 and L3-4 and “lower” for L4-5, L5-S1, and S1-2. Review types were divided into 2 groups: the “chart” (medical record) group (2005–2008), which relied on clinical notes, radiology reports, and an internal photo library, and the “PACS” group (2009–2019), which used picture archiving and communication system (PACS) images for direct review of complete images to assess fusion.
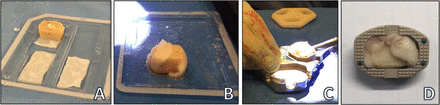
Preparation of anterior lumbar interbody fusion surgical constructs. (A) Hole drilled through femoral head allograft and recombinant human bone morphogenetic protein-2 (rhBMP-2) sponge packed inside. (B) The allograft wrapped in rhBMP-2 sponge. (C) Construct press-fitted inside the polyetheretherketone cage. (D) Cage, allograft, and rhBMP-2.
Surgical hardware was classified into 3 groups based on the type of cage used: “Brantigan” (2005–2009), a PEEK cage with supplementary plate-screw anterior fixation; “STALIF” (2010–2013), a PEEK cage with integrated screws; and “Australis” (2014–2019), a PEEK cage with expanded options for height and lordosis, supplemented with plate-screw anterior fixation. Each standalone cage was packed with a structural femoral head allograft impregnated and wrapped in an absorbable collagen sponge containing a high concentration of rhBMP-2 (Figure 2). No supplemental pedicle screw fixation was applied.
Each patient was treated as a single unit of analysis, resulting in a robust sample size of 1335. This method was chosen to avoid duplicative counting, which would have occurred if the sample had been split into separate levels (n = 1614).
Statistical Analysis
Statistical analysis was performed using R statistical software (version 4.0.2). Descriptive statistics, including mean and SD, were obtained for normally distributed continuous variables, while counts and percentages were used for categorical variables. Exploratory analyses of overall fusion data and fusion rates at 6 and 12 months were conducted using the χ 2 test, with a significance level of 2.05. Age groups, review types, cage types, and ALIF groups assessed differences in proportions.
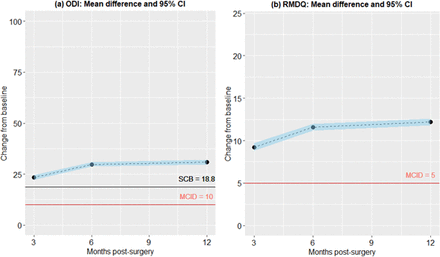
PROMs were summarized using the median and interquartile range at various time points. Changes in VAS-L and VAS-B scores from baseline were estimated using the Hodges-Lehmann estimator. The 95% confidence intervals and P values were derived from a nonparametric sign test. Changes from baseline in ODI and RMDQ were assessed using paired t tests, with mean differences and 95% CIs reported. A Bonferroni correction was applied, with P values less than 0.017 considered statistically significant for each PROM.28
Graphical representations of change scores, along with 95% CIs and the minimum clinically important difference31,32 for VAS-B (12 points), VAS-L (16 points), ODI (10 points), and RMDQ (5 points), were plotted. Substantial clinical benefit (SCB)33 reference lines were included at 25 points for VAS and 18.8 points for ODI.
The effect of subgroups on change scores was examined using mixed-effects regression, with patients as a random factor and time (in months) as the repeated measures factor. Post-hoc comparisons were conducted with Bonferroni correction for multiplicity, with a significance level of 0.05. This approach is robust to slight to moderate residual skewness and can handle missing data.
Results
Table 1 summarizes the demographic characteristics of the 1335 patients. The mean age at the time of surgery was 48.5 years, with most patients (70.9%) in the 40 to 65 age group. Most surgeries were reviewed using PACS images (80.3%) and utilized Australis cages (43.4%). Many cases involved single-level ALIF constructs (80.3%), typically only involving lower levels (96%), with L5 to S1 being the most common level fused (95.4%).
Demographic characteristics of study participants.
Fusion Rates
Regarding fusion rates (Table 2), overall confirmed fusion rates were high, with 1329 patients (99.6%) achieving fusion after surgery. The confirmed pseudoarthrosis rate was 0.2% (3 patients), and 3 patients did not have radiological information available for review.
Fusion rates for 1335 surgery patients.
Most fusions (92.4%) were confirmed by 6 months. Interestingly, of the 1235 patients who had confirmed fusion by the preassigned 6-month postoperative period, 43 patients mistakenly obtained their CT image earlier than expected at 3 months postoperatively but still demonstrated confirmed mature fusion.
A small number of patients (n = 7) had confirmation of their fusion after the 12-month postoperative period. The reasons identified were (1) compliance issues with radiological follow-up (4 patients in total: 2 patients confirmed at 24 months, 1 patient confirmed at 36 months, and 1 patient confirmed at 60 months); (2) documentation issues in medical record notes and radiology reports in the Chart Review Type group (2 patients, confirmed at 24 months); (3) a combination of both compliance and documentation issues (1 patient from the Chart Review Type group, who had confirmed fusion at 48 months).
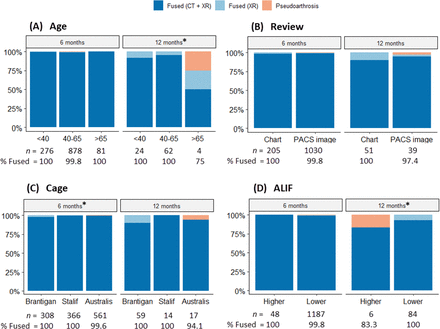
Figure 3 displays fusion rates at 6 and 12 months by age group, review type, cage type, and ALIF group. Notably, patients older than 65 years showed a lower fusion rate (n = 1 of 4; 75% at 12 months) than younger age groups. However, no significant association between age group and fusion rates was found.
Fusion rates at 6 and 12 months by age, review, cage, and anterior lumbar interbody fusion (ALIF) groups. (A) *Lower fusion rate of (n = 1 of 4) 75% at 12 months for the >65 groups compared with other groups: <40 (P < 0.001) and 40–65 (P = 0.014). (B) No significant difference was detected between reviews. (C) *Higher fusion rates (CT + XR) were obtained with STALIF (99.7%) and Australis (99.6%) cages at 6 months than with Brantigan (97.7%). There is no significant difference in overall fusion rates between cage types. (D) *At 12 months, all patients in the Lower ALIF group achieved fusion compared with (n = 1 of 6) 83.3% in the higher group (P < 0.001). CT, computed tomography; PACS, picture archiving and communication system; XR, x-ray imaging.
Association Between Age Group and Fusion
There was no significant association between age group and fusion rates (Figure 3A), with confirmed fusion achieved in 99.7% for those younger than 40 years, 99.6% for those aged 40 to 65 years, and 98.8% for those aged older than 65 years. Comparison through χ 2 analysis was performed: < 40 years vs 40 to 65 years (χ 2 = 0.1, difference = 0.1 [95% CI – 1.4, 0.8], P = 0.80), 40 to 65 years vs >65 (χ 2 = 1.1, difference = 0.8 [95% CI – 0.4, 6.0], P = 0.30), and <40 years vs >65 (χ 2 = 1.1, difference = 0.9 [95% CI – 0.9, 6.1], P = 0.30).
Association Between Review Type and Fusion
Confirmed fusion rates were not significantly different overall between the Chart (98.9%) and PACS image (99.7%) review groups (χ 2 = 3.0, difference = 0.8 [95% CI – 0.1, 2.9], P = 0.085; Figure 3B). However, the proportion of patients for whom fusion was confirmed through CT and XR was 4.1% higher in the PACS image group (99.2%) than in the Chart group (95.1%; χ 2 = 22.4, difference = 4.1 [95% CI – 1.9, 7.4], P < 0.001). Conversely, the proportion of patients for whom fusion was confirmed through XR was only 3.2% higher in the Chart group (3.8%) than in the PACS image group (0.6%; χ 2 = 17.8, difference = 3.2 [95% CI – 1.4, 6.3], P < 0.001). No significant differences were noted between review types at 6 or 12 months after surgery.
Association Between Cage Type and Fusion
Regarding cage type (Figure 3C), no confirmed instances of pseudoarthrosis were observed in either the Brantigan (traditional cage-plate-screw construct) or STALIF cages (integrated screw-cage construct). However, radiological outcomes were not obtainable for 2 patients in the Brantigan group due to the unavailability of PACS imaging at the time, and comments on confirmed radiological reviews were not explicitly defined during the medical record review process.
In the Australis cage group (contemporary cage-plate-screw construct), 3 patients (0.5%) had confirmed pseudoarthrosis. The χ 2 analysis revealed no statistically significant difference in confirmed fusion rates between the Brantigan and STALIF cages (χ 2 = 3.1, difference = 0.8 [95% CI – 0.3, 2.3], P = 0.08), Brantigan vs Australis (χ 2 = 0.3, difference = 0.3 [95% CI – 0.8, 1.9], P = 0.56), and STALIF vs Australis (χ 2 = 1.9, difference = 0.5 [95% CI – 0.6, 1.5], P = 0.17).
Association Between ALIF Group and Fusion
Two instances of pseudoarthrosis were reported in the lower ALIF group and 1 in the higher ALIF group (Figure 3D). The χ 2 analysis found no significant difference between these ALIF groups (χ 2 = 2.5, difference = 1.5 [95% CI – 0.1, 9.5], P = 0.11).
Association Between ALIF Construct Combinations and Fusion
Figure 4 illustrates the fusion rates for single-, 2-, and 3-level ALIF categories at various postoperative time points. In the single-level ALIF construct, 2 patients experienced pseudoarthrosis (1 at L4 and another at L5–S1). Additionally, 1 patient had pseudoarthrosis in a multilevel ALIF construct involving L3 to L4, L4 to L5, and L5 to S1.
Fusion rates in spine surgery patients (n = 1335) were higher than 95% in all available cases, except for the L3–L4/L4–L5/L5–S1 anterior lumbar interbody fusion (ALIF) combination at 12 months, where two-thirds of the group achieved fusion (n = 1 of 3, 66.7%). However, it is noteworthy that all 11 cases with the same combination achieved fusion at 6 months. There were 2 cases of pseudoarthrosis at single levels (L4–L5 and L5–S1) at 6 months (the latter is hard to detect on the graph due to the high percentage of patients achieving fusion). The third case occurred at 12 months in a 3-level ALIF.
Clinical Outcomes
Table 3 and Table 4 show pain and disability PROMs for the entire cohort. All differences from baseline were statistically significant (P < 0.001). The changes from baseline scores were also clinically substantial for all outcomes (Figures 5 and 6). Most scores were above the SCB threshold, except for VAS-L at 3 months. Concerning patient satisfaction, more than 85% reported “excellent” or “good” at all collected time points (Figure 7).
VAS back and leg pain outcomes over 12 months in 1335 surgery patients.
ODI and RMDQ disability outcomes over 12 months in 1335 surgery patients.
Median differences from baseline and 95% confidence interval for visual analog scale (VAS) back and leg pain scores over 12 months after surgery. All median change scores were above the minimal clinically important difference (MCID), and most median scores were above the substantial clinical benefit (SCB).
Mean differences from baseline and 95% confidence interval for Oswestry Disability Index (ODI) and Roland-Morris Disability Questionnaire (RMDQ) disability scores over 12 months after surgery. All mean change scores were above the minimal clinically important difference (MCID) for both measures and above the substantial clinical benefit (SCB) for ODI. No SCB reference was available for RMDQ.
Patient satisfaction levels within 12 months after surgery rated as poor (1), satisfactory (2), good (3), or excellent (4).
Effect of Time, Age, Cage, and ALIF Group on Clinical Outcomes
Linear mixed-effects regression was used to analyze the effect of time (in months) and key subgroups (age, cage type, and ALIF group) on change scores, adjusting for baseline scores. The review type was excluded from multivariable models due to its strong correlation with cage type and its relevance only to the Brantigan cage. Results in Tables 5 and 6 indicate some statistically significant differences; however, the mean differences in improvement between groups at 3 to 12 months after surgery were generally minor and not clinically significant.
Results of the between-groups post-hoc comparisons of the change in pain outcomes after controlling for baseline scores in a multivariable mixed-effects regression model.
Results of the between-groups post-hoc comparisons of the change in disability outcomes after controlling for baseline scores in a multivariable mixed-effects regression model.
Improvements in VAS-B scores were consistent across age groups. Younger patients demonstrated statistically significantly greater improvements in VAS-L scores compared with older patients, suggesting better neurological recovery potential for radiculopathy in younger individuals. Patients younger than 40 years also showed more significant improvements in disability compared with those aged 40 to 60 years, likely due to better recovery potential and less extensive degenerative disease. However, those older than 65 years had statistically comparable improvements in disability to both younger age groups (<40 years and 40–60 years). This may be attributed to differing functional demands associated with periretirement lifestyle changes.
Discussion
This study explored the associations between age, review type, cage type, ALIF group, and fusion rates with standalone ALIF. Fusion rates between the medical record review group (98.9%) and the PACS image review group (99.7%) were similar, though the PACS group had a higher proportion of confirmed fusion through CT and x-ray imaging. Different cage types exhibited varying fusion rates, with the Brantigan cage showing slightly lower fusion rates than the STALIF and Australis cages. In the age subgroup analysis, 12 months postsurgery, patients older than 65 years had lower fusion rates 75% (n = 1 of 4) compared with those younger than 40 years (100%) and those aged 40 to 65 years (100%). At 12 months, the lower ALIF group achieved a 100% fusion rate, compared with 83.3% (n = 1 of 6) in the higher ALIF group, with a significant difference noted (P < 0.001).
Additionally, PROMs for the entire cohort indicated that all baseline differences were statistically significant (P < 0.001). Changes from baseline scores were also clinically significant for all outcomes, with most scores surpassing the SCB threshold,33,34 except for VAS-L at 3 months. These findings highlight age, imaging review methods, and cage types as important factors influencing fusion outcomes, with notable improvements from baseline clinical measures. The combination of structural allograft, BMP-2, and Giannoudis’s Diamond Concept principles may have contributed significantly to this study’s high fusion rate and clinical success.
Standalone ALIF for the treatment of recalcitrant discogenic low back pain secondary to degenerative disc disease offers significant benefits supported by clinical evidence.35–37 Discogenic pain is often associated with radiculopathy.38 The anterior retroperitoneal or transperitoneal approach provides direct access to the disc space, allowing for thorough disc removal and endplate preparation, which creates a more extensive surface area between the bony endplate and the cage and graft. ALIF allows direct and indirect neural decompression, restores disc height and lumbar lordosis, improves spinal alignment in the coronal and sagittal planes, and reduces pain.39,40 By avoiding the posterior approach, ALIF minimizes the risk of damage to the paraspinal muscles, posterior ligaments, and neural elements. The ability to place larger implants improves load distribution and fusion rates, and the anterior approach often results in less blood loss.17
Burkus et al41 investigated Infuse Bone Grafts in single-level ALIF surgeries with threaded cortical allografts. The results showed promising outcomes, including high fusion rates and improved clinical measures, with no reported adverse events related to infuse bone grafts.41 These findings suggest the efficacy and safety of infuse bone graft in facilitating fusion and improving outcomes in this surgical context. Malham et al19 evaluated the use of rhBMP-2 in ALIF procedures with PEEK cages and separate anterior titanium plates. Significant clinical improvements were noted: back pain was reduced by 57.2%, leg pain by 61.8%, and the ODI improved by 54.3%. Additionally, SF-36 scores showed a 41.7% improvement in the physical component summary and a 21.3% improvement in the mental component summary. The study achieved a solid IBF rate of 96.9% at 12 months. Overall, using rhBMP-2 with PEEK cages and anterior titanium plates in ALIF resulted in high fusion rates and significant clinical improvements with a low complication rate.19
Faundez et al20 systematically reviewed clinical data on adverse events linked to the use of rhBMP-2 in ALIF. They found no significant increase in adverse events when rhBMP-2 was used according to Food and Drug Administration guidelines for one-level ALIF with an LT-CAGE Lumbar Tapered Fusion Device.20 Two additional randomized controlled trials reported transient bone remodeling without adverse clinical outcomes. The study concluded that rhBMP-2 is safe and effective in ALIF surgeries when used according to U.S. Food and Drug Administration guidelines, with low complication rates and outcomes comparable or superior to traditional bone grafts.22,23
PEEK or carbon fiber-reinforced PEEK cages in ALIF procedures offer significant biomechanical stability. PEEK promotes fusion due to its favorable biomechanical properties and modulus of elasticity, which closely resembles human bone. Incorporating rhBMP-2 and structural allografts within these cages further supports bone healing and fusion, resulting in high fusion rates and reliable radiographic detection.42 PEEK’s mechanical strength, radiolucency, and biocompatibility make it an ideal biomaterial for ALIF surgeries to facilitate successful IBF.43
This study’s limitations include evolving fusion assessment methodologies, particularly in the pre-PACS era when dynamic x-ray imaging was the accepted standard before adopting postoperative CT evaluation. Although the large sample size is a strength, pseudoarthrosis’s small relative effect size makes it challenging to generalize risk factors for nonunion. The study focused exclusively on standalone ALIF constructs, without comparisons to other IBF approaches or circumferential fusion techniques. By tracking PROMs over time, this case series sheds light on the effectiveness of treatments from the patient’s perspective, offering insights into changes in symptoms, quality of life, and functionality. Despite being limited by the lack of a control group and potential biases, this large case series remains a valuable tool for understanding real-world outcomes, supporting clinical decision-making, and informing future research.
Conclusion
In conclusion, standalone ALIF, particularly when augmented with rhBMP-2 and structural femoral head allografts, provides substantial benefits regarding mechanical stability, higher fusion rates, and clear radiographic assessment. Standalone ALIF is a reliable and effective option for patients requiring lumbar fusion. However, further studies on device design, material properties, and biological enhancements are essential for improving spinal fusion success and achieving positive clinical outcomes.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
Disclosures This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors have no conflicts of interest to disclose.
Ethics approval This study was approved by the University Human Research Ethics Committee (0000015881) of Bond University.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2024 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.