- cervical disc arthroplasty
- motion preservation
- lumbar disc arthroplasty
- interbody fusion
- history of spine surgery
The past 3 decades have brought transformative change in healthcare, with no area more affected than spine surgery. This era has witnessed the rise of interbody fusion from a niche procedure first described by Ralph Cloward in the 1950s to the dominant form of spinal arthrodesis. Other disruptive technologies during this time included the introduction of spinal arthroplasty and minimally invasive surgery as well as the maturation of intraoperative navigation. Innovation in the field of spine surgery is typically the result of an iterative process. It took more than half a century for interbody fusion to become a standard of care procedure, and its impact was not recognized until after Cloward’s death. Similarly, it took decades for intraoperative spinal navigation to become routinely adopted.1,2
Assault on Spine Surgery
I started my neurosurgical residency in July 1992. At that time, a drama was unfolding in the form of the Pedicle Screw Litigation case, which would become an existential threat to spine surgery. In 1992, a cabal of plaintiff’s attorneys filed a host of multimillion-dollar lawsuits against spine surgeons, pedicle screw implant manufacturers, and hospitals. This coordinated legal assault threatened the viability of implant manufacturers and spine surgeons to continue to innovate and push the field into the future.
In early 1993, the US Food and Drug Administration issued warnings to multiple implant manufacturers to stop promoting pedicle screw implants. This promotion basically consisted of training spine surgeons in the proper use of this nascent technology. Later that same year, the ABC News show 20/20 aired a sensationalist segment (“The Secret of the Back Screws,” aired 17 December 1993) reporting on spine surgeons placing “experimental” bone screws, that were not approved for use in the spine, into patients’ backs.3 A witch hunt ensued, fueled by a toxic combination of shoddy journalism, governmental overreach, and plaintiff’s attorneys who filed a multidistrict litigation (class action lawsuit) suing spine surgeons who used pedicle screws as well as spine surgeon societies, implant manufacturers, and hospitals. The lawsuits claimed that these entities promoted the use of devices not approved by the US Food and Drug Administration (FDA) for that explicit purpose.
Ron Pickard, as president/chief executive officer of Sofamor Danek, the largest spinal implant company, and Hansen Yuan, MD, who served as the president of the largest spine society (North American Spine Society) in 1995, stood up to them (Figure 1). They led a coalition of spine surgeons and industry representatives that seized the moral high ground by maintaining that physicians were the true patient advocates. Pickard would remain relentless in his opposition to this litigation. Pickard, who never went to business school or even college, and who started at Danek mowing the lawn and worked his way into the mail room and then all the way up to become chief executive officer, was stalwart in protecting the rights of patients and surgeons. It is not hyperbole to suggest that spine surgery as we know it today would not exist without Pickard’s obstinate leadership against the plaintiff’s attorneys.
Left to right: Ron Pickard; Domagoj Coric, MD; Hansen Yuan, MD; and Jurgen Harms, MD.
Building the Evidence Base
Between 1993 and 1995, several thousand lawsuits related to pedicle screw usage were filed across the United States for hundreds of millions of dollars. In 1997, over the objections of Pickard and Sofamor Danek, Acromed agreed to a $100 million payment to remove them from the class action lawsuit. Pickard vowed to never settle a single case. The FDA requested that a group of spine specialty societies, neurosurgical and orthopedic, develop a retrospective research study to provide an evidence basis for the reclassification of pedicle screws. Ultimately, this research effort culminated in the historical cohort study with Yuan as the lead author.4 I was fortunate to contribute to this effort by reporting on 113 patients from my spine mentor, Charles L. Branch Jr, who had early experience with pedicle screw fixation.5 In 1998, with the evidence-basis provided by the historical cohort study, the FDA formally down-classified pedicle screws from Class III to Class II devices. This action effectively ended the class action lawsuit.3
The Pedicle Screw Litigation case highlighted the need for novel devices and techniques to be evaluated through a critical lens and to be adopted only when a firm evidence-basis for efficacy is established. The positive ramifications of this realization included a commitment to clinical research and outcome data collection that would shape the future of spine surgery. One of the lingering challenges in investigating disruptive technologies is the natural resistance to change and cynicism, which was highlighted by the pedicle screw class action lawsuit. The advent of spinal arthroplasty would encounter the fallout from this controversy.
Founding of SAS/ISASS
The excitement surrounding the introduction of spinal arthroplasty culminated in the founding of SAS in 2000 as a society dedicated to promoting scientific discourse on motion-preserving technologies. Hansen Yuan was a founding member of SAS and served as the society’s president in 2007-2008; he subsequently served as the first editor-in-chief of the SAS Journal, which was later renamed the International Journal of Spine Surgery, until his retirement in 2021. Charles L. Branch Jr succeeded Yuan the International Journal of Spine Surgery editor-in-chief. SAS was renamed ISASS in 2011 with an expanded focus on all innovative spinal techniques.6,7 I am humbled to be part of this academic lineage, serving as ISASS president in 2021-2022.
Motion Preservation Devices
The modern era of spinal arthroplasty was ushered in with the development of the Charité Disc in 1984. The Charité I, as it came to be known, was named after the place in which it was developed, Charité Hospital in East Berlin. Like celebrities with 1-word monikers, the Charité has an interesting backstory. Its inventor was Karin Büttner-Janz, a world-class gymnast who won 2 gold medals for East Germany in the 1972 Olympic Games in Munich. After her gymnastic career, Büttner-Janz became an orthopedic surgeon and, eventually, president of the Spine Arthroplasty Society (SAS), the precursor society to the International Society for the Advancement Spine Society (ISASS). In 2004, the Charité III lumbar total disc replacement (L-TDR) became the first FDA-approved spinal arthroplasty device. The Charité III was met with considerable fanfare and even more controversy related to numerous factors, including surgical indications and potential complications related to L-TDR.
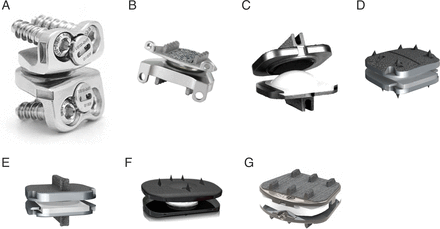
The first modern cervical total disc replacement (C-TDR) device was described by Cummins in Bristol, United Kingdom, in the late 1980s. It was an entirely stainless steel design consisting of a metal-on-metal ball and trough device with a faceplate attached via anchoring screws.8 High failure rates led to the redesigned Frenchay disc, which would ultimately become the first FDA-approved cervical disc as the Prestige ST (Figure 2A).9 The modern incarnation of this disc, the Prestige LP (Figure 2B), is still marketed today. Another first-generation C-TDR device, the Prodisc-C (Figure 2C), designed by Thierry Marnay (another SAS president), utilized a ball and socket articulation with cobalt-chrome alloy keeled endplates and a polymer core (ultra-high molecular weight polyethylene). In 2023, 2 next-generation Prodisc-C devices, Vivo and SK (Figure 2D and E), received regulatory approval.
Cervical artificial discs: (A) Prestige ST, (B) Prestige LP, (C) Prodisc-C, (D) Prodisc C Vivo, (E) Prodisc C SK, (F) Baguera-C, and (G) Synergy.
The initial exuberance surrounding L-TDR led to the development of several C-TDR devices as well as posterior lumbar facet replacement devices. These devices took divergent paths to regulatory approval and clinical adoption. L-TDR languished due to clinical controversies as well as limited insurance coverage and reimbursement issues despite FDA approval. Since the original Charité III (2004) and Prodisc-L (2005) FDA approvals in the early 2000s, 5 L-TDR devices entered the investigational device exemption (IDE) trial, but only 1 device, activ-L, received approval (2015). The posterior facet replacement devices struggled to clear regulatory hurdles and complete the IDE process. In 2005, the Total Facet Arthroplasty System (TFAS Device) became the first lumbar facet replacement device to enter into the FDA IDE study. Shortly afterward, the TOPS Device (2005) and the ACADIA Facet Replacement Device (2006) also entered FDA IDE study. None of these devices successfully navigated the IDE process. The original TOPS device was revised and re-entered IDE study in 2017, ultimately receiving the first FDA-approval for a posterior lumbar arthroplasty device in 2023. Conversely, C-TDR has been the success story of spinal arthroplasty.
C-TDR gained widespread acceptance and adoption on the strength of the unprecedented scientific evidence basis for its efficacy. Since the FDA approval of the Prestige-ST in 2006, there have been a total of 11 FDA-approved C-TDR devices. An additional 2 cervical artificial discs, Baguera-C and the Synergy Disc (Figure 2F and G), have completed IDE study enrollment and remain active in IDE regulatory process. There have been more than 4000 patients prospectively studied at nearly 300 IDE study sites with hundreds of individual investigators. More than 14 prospective IDE trials (levels I and II evidence) with long-term follow-up of 5 to 10 years have been published. Each of these devices has shown at least noninferiority to a high bar “gold standard” of anterior cervical discectomy and fusion. This preponderance of high-level data, replicated with several different devices, led to regulatory approval followed by insurance coverage and, ultimately, increasing clinical adoption and usage.10–14
There are currently 16 FDA-approved spinal arthroplasty devices (12 C-TDR; 4 lumbar: 3 L-TDR and 1 lumbar posterior facet replacement device). There are an additional 3 spinal arthroplasty devices (2 C-TDR; 1 lumbar posterior disc/facet hybrid device, MOTUS) in active IDE study that have completed IDE pivotal trial enrollment. Six spinal arthroplasty devices (2 C-TDR and 4 L-TDR) that completed IDE enrollment but were never submitted for FDA approval are no longer in IDE study.
There are several compelling reasons that have prevented L-TDR from achieving the popularity of C-TDR. There is inherent difficulty in diagnosing, and controversy in treating, the primary indication for L-TDR, namely, discogenic low back pain (LBP). The etiology of axial LBP is heterogeneous with multiple potential pain generators (eg, disc, facets, basivertebral nerve, SI joint, musculoskeletal, and rheumatological conditions). Furthermore, diagnostic tests have not reliably correlated radiographic abnormalities with clinical symptoms of axial LBP. Provocative discography is invasive and has largely fallen out of favor. Magnetic resonance imaging does not provide a proven clinical correlation, and promising alternatives, such as magnetic resonance spectroscopy, have not yet been validated. The controversy surrounding the surgical treatment of LBP itself further impeded the adoption of L-TDR. LBP is a ubiquitous condition that does not threaten neurological function and, in the vast majority of cases, does not require surgical consideration. Those cases that do progress to surgery have traditionally been treated with a significant procedure: arthrodesis. Overall success rates are dramatically lower for the surgical treatment of LBP (L-TDR = 50%–60%; lumbar fusion = 40%–55%) compared with cervical radiculopathy (C-TDR 85%–95%; anterior cervical discectomy and fusion 70%–85%), the primary indication for C-TDR, or lumbar radiculopathy and neurogenic claudication (artificial facet = 93%; transforaminal lumbar interbody fusion [TLIF] = 81%), the indications for facet replacement. Additionally, L-TDR necessitates an anterior retroperitoneal approach, which is relatively more precarious compared with the more common posterior approach to the lumbar spine, and requires an exposure surgeon.
Unlike L-TDR, posterior lumbar arthroplasty utilizes the traditional posterior midline exposure, the workhorse approach to the lumbar spine, which is familiar to virtually every spine surgeon. Posterior lumbar arthroplasty’s primary indication is stenosis secondary to spondylolisthesis presenting with neurogenic claudication and/or radiculopathy. Unlike with LBP, it is generally accepted that medically refractory spondylolisthesis/stenosis is a viable indication for surgery, including fusion. Controversy does exist regarding whether decompressive laminectomy vs decompression and fusion is the appropriate procedure for spondylolisthesis. In fact, decompression with dynamic stabilization may represent a compromise between these extremes. Therefore, like C-TDR in the cervical spine, posterior artificial facet replacement utilizes the workhorse approach to the spine and treats neural compression.15–19
A new era in spinal arthroplasty began with the first FDA-approved posterior lumbar facet arthroplasty device. Statistically superior results compared with the standard fusion procedure, TLIF, were reported at 24 months, leading to an FDA-approved superiority designation over TLIF. A second posterior lumbar arthroplasty, MOTUS, has already completed enrollment of its IDE pivotal trial and is actively seeking FDA approval. Continued focus on posterior lumbar arthroplasty treating neural compression from a traditional approach is more likely to lead to more widespread adoption compared with L-TDR. Recently, L-TDR has enjoyed something of a renaissance as well. This revival has been driven by positive long-term (up to 10 years) clinical results as well as a novel FDA indication of a 2-level approval (2022). The steady pipeline of next-generation C-TDR devices continues the evolution of this procedure with improved biomaterials and biomechanics. Modern artificial discs are composed of radiolucent materials, such as titanium and polyetheretherketone, with improved intra- and postoperative visualization and imaging. Incremental refinements in biomechanical characteristics have resulted in devices that more closely mimic natural motion.15,18,20–22
Conclusion
The field of spine surgery in the 1990s was marked by adversity in the form of the Pedicle Screw Litigation case. Spine surgeons, both neurosurgical and orthopedic, coalesced with implant manufacturers to successfully battle this existential threat with a combination of steely resolve and scientific evidence. Innovation in the spine field over the ensuing decades was colored by this experience. Spinal arthroplasty in general and C-TDR in particular underwent unprecedented scientific scrutiny and emerged with an unparalleled evidence basis for efficacy. Emerging spinal technological advances, such as robotic spine surgery and disc repair, should be subjected to the same crucible. SAS, now known as ISASS, exemplifies professional societies that serve as safe havens for professionals dedicated to the advancement of spine science. They encourage collaboration as well as the free exchange of ideas and discourse related to innovative techniques that are necessary for vetting novel technologies and improving patient care.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures and Conflicting Interests Domagoj Coric reports royalties/licenses from Spine Wave, Acellus, Medtronic, and Globus; consulting fees from Spine Wave, Medtronic, and Globus; payment/honoraria from Spinal Elements; support for attending meetings/travel from Spinal Elements, Medtronic, and Globus; serving on the AANS Board of Delegates; and stock/stock options for Spine Wave, Premia Spine, and 3Spine.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2025 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.