ABSTRACT
Background: The purpose of the present study is to report the 2-year clinical outcomes for chronic low back pain (CLBP) patients treated with radiofrequency (RF) ablation of the basivertebral nerve (BVN) in a randomized controlled trial that previously reported 1-year follow up.
Methods: A total of 147 patients were treated with RF ablation of the BVN in a randomized controlled trial designed to demonstrate safety and efficacy as part of a Food and Drug Administration-Investigational Device Exemption trial. Evaluations, including patient self-assessments, physical and neurological examinations, and safety assessments, were performed at 2 and 6 weeks, and 3, 6, 12, 18, and 24 months postoperatively. Participants randomized to the sham control arm were allowed to cross to RF ablation at 12 months. Due to a high rate of crossover, RF ablation treated participants acted as their own control in a comparison to baseline for the 24-month outcomes.
Results: Clinical improvements in the Oswestry Disability Index (ODI), Visual Analog Scale (VAS), and the Medical Outcomes Trust Short-Form Health Survey Physical Component Summary were statistically significant compared to baseline at all follow-up time points through 2 years. The mean percent improvements in ODI and VAS compared to baseline at 2 years were 53.7 and 52.9%, respectively. Responder rates for ODI and VAS were also maintained through 2 years with patients showing clinically meaningful improvements in both: ODI ≥ 10-point improvement in 76.4% of patients and ODI ≥ 20-point improvement in 57.5%; VAS ≥ 1.5 cm improvement in 70.2% of patients.
Conclusions: Patients treated with RF ablation of the BVN for CLBP exhibited sustained clinical benefits in ODI and VAS and maintained high responder rates at 2 years following treatment. Basivertebral nerve ablation appears to be a durable, minimally invasive treatment for the relief of CLBP.
INTRODUCTION
Low back pain (LBP) is the most expensive occupational disorder in the United States and the leading cause of disability worldwide.1–3 There are no validated diagnostic reference standards for LBP, leading 85% of patients to be diagnosed with so-called nonspecific LBP.4–6 Absent a specific diagnosis, chronic LBP (CLBP) patients are treated with an assortment of costly interventions, including medications (opioid and nonopioid), physical therapy, manipulative therapy, massage therapy, bracing, and injections. Few of these therapies have Level I evidence supporting their effectiveness in the CLBP population. Eventually, a large number of these patients seeking pain relief undergo fusion surgery with its associated long rehabilitation time,7 modest outcomes,8,9 high costs,10–13 high complication rates and 2- to 4-year reoperation rates of 20%.13,14 The chronic nonspecific nature of CLBP also results in many individuals being prescribed opioid analgesics, whether or not they eventually undergo fusion surgery.15–18
Notwithstanding the nonspecific LBP descriptor, spine care practitioners have historically presumed that the intervertebral disc was the source of most CLBP.19–21 The discogenic LBP model holds that sensitized nociceptors within the posterior annulus of degenerating discs cause LBP. However, anatomic studies have shown that the vertebral endplate is actually the source of most of the pathologic innervation that occurs with disc degeneration. These studies suggest that, in some patients, the origin of LBP is the vertebral endplates, with pain transmitted via the basivertebral nerve (BVN).22–24
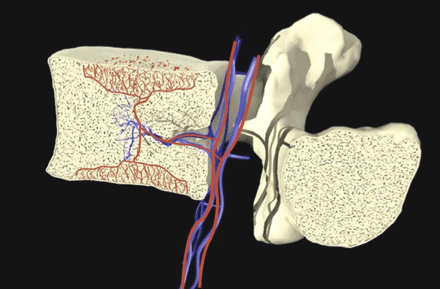
The BVN bundle, often in the form of paired nerves, originates from the sinuvertebral nerves and enters the vertebral body posteriorly via the central vascular foramen. It courses centrally within the vertebral body, generally following the basivertebral vessels and sending branches towards the endplates.25,26 An illustration of the BVN and vasculature anatomy is provided in Figure 1. Immunohistochemical analysis showed that the BVN contains Substance P, CGRP, and PGP 9.5 positive nerve fibers, substantiating the nerve's nociceptive role in the conduction of vertebrogenic pain signals in CLBP individuals.27–29
Basivertebral nerve (red) and vasculature (blue).
Compared with the density of nociceptors in normal endplate regions or in painful degenerated discs, BVN density is much higher in endplate regions with damage, linking these nerves to CLBP.22 As the disc and endplates degenerate, the communication between the bone marrow and the disc increases, due to the hydraulic disc/vertebra coupling and increased convective flow induced by cyclic spinal loading. This crosstalk that develops between the disc and bone marrow results in the release of inflammatory mediators. The persisting stimulus sets up a frustrated healing response, leading in some individuals to an escalating inflammatory response and the appearance of Modic changes (MCs) at the vertebral endplates.23,24,30 This inflammatory response is sensed by the BVN and transmitted to the central nervous system, then perceived as LBP.
Provocative discography has been used in the past to diagnose discogenic LBP. However, discography is currently controversial with some studies claiming a high false positive rate in certain populations and associating discograms with accelerated disc degeneration.31,32
Additionally, discography has been shown to result in deflection of the endplates with the suggestion that a positive discogram might be the result of pressurization of painful vertebral endplates.33
In contrast, vertebrogenic pain is diagnosed using MCs that are readily identifiable on routine MR imaging. Modic type endplate changes represent a classification for vertebral body endplate magnetic resonance imaging (MRI) signal, first described in 1988.34 Modic Type 1 and 2 changes are associated with degenerative disc disease and LBP.35 Studies have also shown that CLBP patients with MC are clinically different than CLBP patients without MC.36 Patients with MC report a greater frequency and duration of LBP episodes and seek care more often.37 Additionally, CLBP patients with Type 1 MC have poor outcomes relative to conservative treatment38,39 and have worse outcomes after discectomy.40
Utilizing the scientific foundations of a vertebrogenic origin for CLBP, a transpedicular, minimally invasive procedure using radiofrequency (RF) energy to ablate the BVN near the center of the vertebral body was developed. It was hypothesized that, once ablated, these nerves would no longer transmit pain signals. To test this theory, a 225-patient, Level I randomized controlled trial comparing treatment of CLBP with RF ablation of the BVN to a surgical sham control was performed. The initial, previously published results from this study demonstrated that intraosseous RF ablation of the BVN is a safe and effective treatment for CLBP in patients with Modic Type 1 or 2 abnormalities of the endplates.41 This study, in addition to the pilot study results, validated the use of MCs as a diagnostic criterion for vertebrogenic pain. We report on the 2-year clinical results for patients treated with RF ablation of the BVN in this study.
MATERIALS AND METHODS
Study Design
The original trial was a prospective, randomized, sham-controlled, double-blind, pivotal Food and Drug Administration (FDA) Investigational Device Exemption (IDE) clinical trial (SMART Trial ClinicalTrials.gov—NCT01446419). The trial was conducted at 15 sites (202 patients) in the United States and 3 sites (23 patients) in Europe. The primary requirements for inclusion in the trial were CLBP with a duration greater than 6 months, CLBP nonresponsive to at least 6 months of nonsurgical management, and Modic Type 1 or 2 changes at the vertebral endplates of the levels targeted for treatment. Detailed information about the study design and randomization process were previously published.41 Primary inclusion and exclusion criteria are listed in Table 1.
Inclusion and exclusion criteria.
Study Interventions
Patients were randomized 2:1 to either a RF ablation procedure or a sham control procedure. The RF ablation procedure consisted of thermal ablation of the BVN using a transpedicular delivery system (Intracept System, Relievant Medsystems, Sunnyvale, CA, USA). The Intracept procedure was performed under image guidance in an outpatient setting with thermal ablation for 15 minutes to create approximately a 1 cm spherical lesion within the vertebral body. Detailed information about the surgical technique was previously described.41 Patients in the sham arm underwent the same operating room protocol for the same overall duration of 60 to 90 minutes; however, the sham procedure consisted only of docking the introducer cannula 1–2 mm into the pedicle and simulating the RF ablation with an equivalent dwell time. To maintain blinding, the treating and follow-up physicians differed, and the patients were unaware of the randomization outcome for 1 year. All participants continued conservative therapy as prescribed at baseline. Additional medical therapies could be prescribed per the blinded follow-up investigator's discretion. For patients in the sham arm, there was an optional crossover component after all 12-month evaluations were performed.
Outcome Measures
Outcomes were evaluated using the Oswestry Disability Index (ODI) questionnaire and the Medical Outcomes Trust Short-Form Health Survey (SF-36). Back pain was assessed using a visual analog scale (VAS) on a 10-cm scale ranging from 0 (no pain) to 10 (worst pain imaginable). Physical and neurological examinations were performed at each follow-up visit. Patients attended follow-up visits at 2 and 6 weeks, and 3, 6, and 12 months during the first year of follow up.
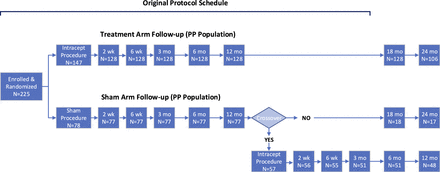
The protocol was subsequently modified to include longer-term follow up to 24 months. During the second year, the treatment arm patients were followed for safety only at 18 months, and at 24 months for ODI, VAS, SF-36, as well as safety. Sham arm patients who did not choose to cross over to Intracept RF ablation treatment were also followed per this schedule. Participants who elected to cross to RF ablation were followed at 2 and 6 weeks, and 3, 6, and 12 months postprocedure. Of the 78 patients in the sham arm, 57 (73%) elected to cross over to receive the Intracept treatment. A flowchart that reflects study design and patient disposition is provided in Figure 2.
Study design and patient disposition.
Magnetic resonance imaging (T1, T2, and STIR time constants) was performed at 6 weeks and 6 months after the index procedure. Targeting success for treatment was confirmed by an independent, blinded radiologist reviewer and was defined as overlap between the RF-created ablation zone and the terminus of the BVN at each level treated observed on 6-week MRI.
Statistical Methods
Two preplanned analysis groups were defined within the FDA approved IDE protocol which governed the original study. These were the intent-to-treat (ITT) and the per-protocol (PP) populations. The ITT population consisted of the patients as randomized (ITT population: treatment arm n = 147, sham arm n = 78). As the study entailed randomization to a surgical procedure, the possibility of an unsuccessful procedure was admitted, with the most likely cause being failure of the RF generated lesion to be collocated with the terminus of the BVN. The PP population excluded patients that did not meet the minimum treatment requirement (incomplete procedures n = 1, failed targeting success n = 16) and 2 patients who were not adherent to the postprocedure protocol (PP population: treatment arm n = 128, sham arm n = 77). Detailed reasons for patients excluded from the PP population were previously reported.41 The 24-month demographic and safety results are reported for all treated patients; efficacy results are reported for the PP population who completed a 24-month evaluation.
Seventy-three percent of the sham patients elected to cross over to active treatment after 12 months of follow up; thus, a 24-month follow-up statistical comparison between arms cannot be performed due to a lack of statistical power. Therefore, an intrapatient comparison of patient-reported measures from baseline to each follow-up visit was performed to evaluate the long-term efficacy and treatment durability in RF ablation arm participants with 24-month data. The efficacy measures are summarized using descriptive statistics (eg, N, mean, standard deviation). Comparisons between the postoperative and baseline values were performed using a paired t test.
RESULTS
Demographics
Out of the 225 patients included in the original study, 147 patients were treated with RF ablation of the BVN and 78 with the sham control procedure. Patient characteristics between the 2 arms were previously reported as similar.41 Baseline information about the patients treated with RF ablation is listed in Table 2.
Patient demographics and baseline characteristics.
Treatment Success
All procedures were completed in the treatment arm except for 1 patient with extremely dense bone at S1. Targeting was adjudicated via imaging by a blinded independent radiologist and was deemed successful in 129 of 145 patients (89.0%), or in 300 of 317 treated vertebral bodies (94.6%). The most commonly treated levels were L5–S1 (85/146 patients) and L4–L5 (29/146) followed by L4–L5–S1 (23/146). An additional 5 patients were treated only at L3–L4 with 4 patients treated at L3–L4–L5.
Oswestry Disability Index
The primary efficacy endpoint for the original study was the 3-month change in ODI compared between the study arms. This comparison, as previously reported,41 found that at 3 months the PP treatment group exhibited a 20.5 least squares mean (LSM) improvement in ODI compared to a 15.2 LSM improvement in the sham group (P = .019).
Of the PP population treated with BVN ablation, 106 patients completed a 24-month follow-up visit. These patients exhibited a durable ODI mean improvement (23.4 points) at 24 months compared to the mean improvements observed during their first year of follow up (20.3, 20.8, and 19.8 points at 3, 6, and 12 months, respectively). In terms of percent improvement in ODI from baseline, these results translate into mean percentage improvements of 53.7% at 24 months compared to 47.6, 48.2, and 46.2% at 3, 6, and 12 months, respectively. Oswestry Disability Index outcome results for all time points are shown in Table 3.
Patient reported outcomes by visit.
Using a 10-point improvement in ODI, the commonly accepted minimum clinically important difference (MCID) in the treatment of CLBP,42,43 75.6% (96/127) of treated patients exhibited a successful response at 3 months. At 24 months, this result was sustained with 76.4% (81/106) of treated patients continuing to receive clinical benefit from BVN ablation. When considering a 20-point threshold for reduction in ODI, 48.0% (61/127) of treated patients showed such an improvement at 3 months and 57.5% (61/106) at 24 months.
Visual Analog Scale
The mean VAS pain score reported at baseline was 6.73 cm and improved by 2.76 and 3.59 cm at 12 and 24 months of follow up, respectively (see Table 3). When considering the results from the perspective of percentage improvement from baseline, the mean percent improvement in VAS at 24 months was 52.9 %, compared to 42.8, 44.2, and 40.1% at 3, 6, and 12 months, respectively.
At the primary 3-month time point, 65.4% (83/127) of patients met or exceeded the VAS MCID of 1.5 cm improvement.42 This VAS responder rate was sustained with 70.2% (73/104) of patients meeting or exceeding the 1.5 cm improvement in VAS at 24 months.
Medical Outcomes Trust Short-Form Health Survey Physical Component Summary
The mean SF-36 Physical Component Summary (PCS) score reported at baseline was 33.50, which improved to 42.83 and 45.83 at 12 and 24 months of follow up, respectively. The mean improvement in the PCS score observed at 24 months was 11.84 and showed durability compared to the other study time points. The MCID for the PCS has been reported as 4.9.44 The SF-36 PCS results are reported for all study time points in Table 3.
Resource Utilization
Baseline characteristics of the PP patients who were treated with RF ablation and followed at 24 months (n = 106), showed that 68 (64.2%) of the patients had pain for more than 5 years, and 28 (26.4%) were taking opioids. Additionally, the underlying incidence of patients with previous spinal injections was 61 (57.5%).
Utilization of opioids was monitored at all follow-up time points through the first year of the trial. During the second year of follow up, documentation of opioid usage was not reliably available due to the infrequency of patient visits. In the above population, at 12 months of follow up, 60.7% of the patients who were taking opioids at the time of enrollment had reduced opioid medication, with 46.4% completely eliminating the use of opioids.
During the first year of follow up, the study protocol discouraged physicians from treating patients with spinal injections unless medically necessary. Following unblinding of patients after the 1-year study visit, patients were managed per standard clinical practice, and thus, the rate of interventions seen during the second year of follow up would no longer be expected to be biased by study design. At 24 months of follow up, in PP patients treated with ablation, 8 (7.5%) had spinal injections performed for either new onset or continuing LBP, a substantial decrease from the 61 (57.5%) who had reported prior injections at baseline.
Complications/Interventions
Adverse events (AEs) were continuously assessed during the study and reported as they occurred. Each AE was assessed by the study investigator for severity and relationship to the study device or procedure. An independent review of clinical trial execution and safety data was performed on a regular basis by an external data safety monitoring board (DSMB) throughout the course of the trial.
There were no device or procedure-related patient deaths, no unanticipated adverse device effects, and no device-related serious adverse events (SAEs) reported in the study. One device- related AE occurred in a sham patient, who crossed over to the active treatment at 1 year. The patient was being treated with high levels of hormone replacement therapy and developed a vertebral compression fracture. Further diagnostic workup revealed concomitant osteopenia. By 8 weeks, the fracture was healed with no further complications. Procedure-related events following the index procedures were minimal, and MRI evaluations at the 6-week and 6-month follow-up time points found no evidence of any spinal cord abnormalities, avascular necrosis, or accelerated disc degeneration (these results were previously reported).41
During the first year of follow up, 2 RF ablation-treated patients underwent surgical spine interventions. One was a lumbar fusion at the same level that was treated in the index procedure performed due to continuing LBP; the second procedure was a microdiscectomy performed due to a de novo disc herniation at the L2–L3 level, which was not the same level that was treated in the index procedure (index procedure was performed at L4–L5).
During year 2, 8 RF ablation treated patients exited the study to undergo other surgical therapies on the lumbar spine. Seven of these were lumbar fusion procedures performed at the same levels as the index procedures. It should be noted that 5 of these 7 procedures were performed by 1 investigational site following unblinding of the patients at the 12-month follow-up visit. The remaining procedure was a discectomy following an acute foraminal disc herniation that occurred at a different level than what was targeted in the index procedure.
DISCUSSION
There has been extensive direct evidence supporting the vertebral body as a source of LBP (ie, vertebrogenic pain) reported in the published literature.22,33,45–48 Additionally, the BVN within each vertebral body has been shown to be a source of transmission of vertebrogenic pain signals in CLBP individuals.25,26 Furthermore, correlation between vertebral body pathology (as demonstrated by MCs) and CLBP has also been reported.34,40–52 The SMART trial was designed to test the hypothesis that the BVN plays an important role in the transmission of pain signals in patients with CLBP and exhibiting Modic Type 1 and 2 endplate changes. Initial results of the SMART trial showed that thermal ablation of the BVN is a safe and effective treatment for CLBP patients with this indication.41
Longer-term results for patients who were treated with RF ablation show that the clinically meaningful improvements in ODI, VAS, and SF-36 PCS observed at 1 year are sustained through 2 years of follow up and are more than double the MCID for all measures. The mean percentage improvements in ODI and VAS compared to baseline at 2 years were 53.7 and 52.9%, respectively. Responder rates for ODI and VAS were also maintained through 2 years with patients showing clinically meaningful improvements in both: 76.4% of patients had ≥10-point ODI improvement and ODI ≥ 20-point improvement in 57.5%; 70.2% of patients had ≥1.5 cm VAS improvement.
The original study design compared results reported from patients treated with RF ablation to those treated with a sham control procedure through 12 months of follow up. After 12 months, all patients in the study were unblinded as to which treatment they had received. Sham patients were subsequently offered the opportunity to cross over to receive the RF ablation procedure, of which 73% elected active treatment. Comparisons between the original study arms could therefore not be performed for long-term evaluation of efficacy outcomes as the untreated, control population was too small for adequate statistical power.
Comparison to the crossover population was also not appropriate, as the patient-reported outcomes of this population at the time of crossover were no longer consistent with the original baseline status. The sham population experienced a large placebo response, as was discussed in the prior publication of the 1-year study results.41 Therefore, for the purpose of long-term evaluation of patient-reported outcomes, postoperative results were compared to baseline (preoperative) measurements, with each treated patient serving as their own control, consistent with other spine procedure trials.
Patient retention through 1 year of follow up in the study was excellent with 97.7% (125/128) patients in the PP treatment population evaluated at 12 months. Reasons for nonevaluation included 1 patient death (not study related) and 2 patients who exited the study early to undergo surgical spine therapies. At 2 years, 84.8% (106/125) of the 12-month PP treatment group returned for follow up. Reasons for nonevaluation at 2 years included 4 patients who withdrew consent and 6 patients who exited the study due to surgical spine procedures. An additional 9 enrolled patients failed to return for evaluation at 24 months.
Due to the overall 17% PP patient fallout by 24 months, we looked at the mean ODI and VAS measures for the PP-treated population at 24 months (n = 106) compared to the overall PP treated population (n = 128) at baseline and 12 months to ensure the PP-treated population evaluated at 24 months was not unintentionally biased. The baseline PP-treated patient reported outcomes were nearly identical for the 2 populations; the mean ODI and VAS in the 24-month PP-treated population were 42.2 and 6.72 cm, respectively, compared to 42.4 and 6.73 cm for the overall PP-treated population. At 12 months, the populations remained comparable; the mean ODI and VAS in the 24-month PP-treated population were 20.8 and 3.84 cm, respectively, compared to 22.6 and 3.96 cm for the overall PP-treated population. Thus, we believe the PP-treated population evaluated at 24 months is reflective of the enrolled PP treatment population.
The incidence of spine-related surgery in the ITT RF ablation-treated patients over the 2-year study period (6.8%, 10/146) in this trial is comparable to other studies reported in the literature for patient populations with degenerative disc disease. Numerous studies have shown that the extent of disc degeneration and disc herniation increases over time as part of the natural aging process. In studies examining asymptomatic patients, de novo herniations or progression of existing disc herniations were observed in 11.4% of patients after 3 years,53 24.4% of patients after 5 years, and 32.3% of patients after 7 years.54 Additionally, in patients with a history of disc degeneration and LBP, the percent values of disc herniation were as high as 78% after 17 years.55 These studies also showed that the rate of deterioration of disc status is increased in patients who have a history of disc degeneration and/or herniation. Thus, the observed incidence of progression of existing disc degeneration and herniation seen in this study of patients with CLBP is not unexpected given the published occurrence rates.
In this study, of the 10 patients that underwent spine surgery over the 2-year study period, 2 had removal of symptomatic disc herniations at nontreated levels. Of the 8 patients that underwent surgery after 1 year, 7 progressed to lumber fusion; however, these were disproportionately performed at a single study site. Thus, the incidence of lumbar fusion post-BVN ablation may be overstated in this trial's results and not reflective of future outcomes.
Prior CLBP studies of other treatment modalities have noted that improvements in pain gradually deteriorated after 6 months.8 These 24-month study results support the longevity of BVN ablation. Radiofrequency ablation of the BVN is due to thermal damage of tissue proteins within the zone of coagulation adjacent to the Intracept probe tip. Coagulation is surrounded by a secondary zone of hyperemia, where there is local release of inflammatory factors, edema, and changes in blood flow.56
These concentric zones result in the characteristic bull's eye appearance of the Intracept lesion seen in clinical MRI images. Histologically, blood vessels within the zone of coagulation are embolized, nerves undergo Wallerian degeneration, and neural tissues disintegrate.57 Unpublished histological studies from a large animal model demonstrate that, by 12 months: (1) hematopoietic marrow in the zone of coagulation is replaced by viable fat; (2) new bone is formed on preexisting trabeculae. There was no evidence of avascular necrosis; and (3) rudimentary blood vessels and nerves develop at the coagulation zone periphery.58 The BVN itself does not appear to regenerate, which may be due to the extent of the thermal injury combined with the fact that the BVN is nonmyelinated, leading to a more comprehensive and permanent nerve destruction.59,60
SUMMARY
Radiofrequency ablation of the BVN is a safe and effective minimally invasive treatment for the relief of CLBP in patients with Type 1 or 2 MCs. Patients treated with RF ablation of the BVN for CLBP exhibited sustained clinical benefits in ODI and VAS and maintained high responder rates at 2 years after treatment. This therapy reported statistically significant improvements from baseline at all time points through 2 years of follow up. Basivertebral nerve ablation appears to be a safe, durable, minimally invasive treatment for the relief of CLBP in a patient population that typically has few effective treatment options.
Footnotes
Disclosures and COI: Rick Sasso, Scott Kitchel, Hyun Bae, Christopher Yeung, Eeric Truummees, Philip Yuan, Peter Vajkoczy, Michael DePalma, and Lee Thibodeau have no disclosures to report. Jeffrey S. Fischgrund reports consultancy for Relievant. Alfred Rhyne reports consultancy for Relievant. Jörg Franke reports consultancy and an honoraria from Relievant. Michael Schaufele reports consultancy for Relievant. David G. Anderson reports consultancy for DePuy Synthes Spine, K2M Spine, and Integrity Spine. Bernhard Meyer reports honoraria, travel support, consultancies, and funded research by Medtronic, DePuy, Umrich, Icotech, Spineart, and Relievant. Research Oversight and Ethics: This research was conducted under the oversight of the Western Institutional Review Board and the investigational site's local institutional review board. Informed consent was obtained for participants in this study. This research was conducted in accordance with the Helsinki Declaration.
- ©International Society for the Advancement of Spine Surgery
- Copyright © 2019 ISASS - This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery