Abstract
Background Numerous studies have confirmed that both posterior lumbar interbody fusion (PLIF) and posterior lumbar fusion (PLF), have their advantages and disadvantages. However, the inconsistent results of these studies make it difficult to reach a consensus on which fusion method is superior.
Objective To compare the clinical outcomes of PLIF, PLF, and hybrid surgery combining PLIF and PLF in the treatment of lumbar degenerative disease.
Methods A retrospective review was conducted, collecting clinical records and radiological data of patients with lumbar degenerative disease from 2014 to 2022. Patients were divided into 3 groups based on surgical strategy: PLIF group, PLF group, and hybrid group. Clinical data included patient-reported outcomes such as the Japanese Orthopedic Association score, Oswestry Disability Index score, visual analog scale score, 36-item Short Form Health Survey score, and the occurrence of complications. Radiological data included Cobb angle, fusion rate, adjacent segment degeneration (ASDeg), adjacent segment disease (ASDis), and cage subsidence.
Results A total of 378 patients were divided into 3 groups: PLIF group (n = 122), PLF group (n = 126), and hybrid group (n = 130). The baseline characteristics were balanced among the 3 groups. As the follow-up time increased, visual analog scale scores showed varying degrees of improvement (all P measure time < 0.001), but there were no significant differences observed between the groups (all P measure time * group > 0.05). Oswestry Disability Index scores improved over time (F measure time = 939, P measure time < 0.001), with the hybrid group showing more significant improvement (F measure time * group = 2.826, P measure time * group = 0.006). The 36-item Short Form Health Survey scores and Cobb angles also improved significantly during the follow-up period, with no significant differences observed among the groups. The overall fusion rates for the hybrid group and PLIF group were 93% and 91%, significantly higher than the fusion rate of the PLF group (84%; P = 0.031). The postoperative complication rate was significantly higher in the PLIF group (24.4%) compared with the PLF group (16.4%) and the hybrid group (12.5%; P = 0.022). There was no significant difference in the overall 5-year ASDeg occurrence rate (38% vs 36%) and ASDis occurrence rate (11.3% vs 8.3%) between the PLIF group and PLF group for single-level fusion (P > 0.05). The occurrence rate of ASDeg for multilevel fusion in the hybrid group was 29%, significantly lower than that in the PLIF group (42%) and PLF group (37%; P = 0.044). The overall 5-year ASDis occurrence rates for multilevel fusion were 12.3%, 9.9%, and 7.6% for the PLIF group, PLF group, and hybrid group, respectively, with no significant statistical difference (P = 0.338).
Conclusion All 3 surgical techniques might improve the clinical symptoms of patients with degenerative lumbar disease effectively. The hybrid technique demonstrated comparable efficacy to PLIF and PLF in increasing fusion rate, reducing complications, and decreasing the occurrence of ASDeg in multilevel fusion cases significantly.
Clinical Relevance This study holds significant clinical relevance as it directly addresses the treatment outcomes of common surgical interventions for lumbar degenerative disease, a condition that significantly impacts patient quality of life and functionality. This study is also crucial for clinicians when selecting the most appropriate treatment strategy for patients with lumbar degenerative disease.
Level of Evidence 3.
- lumbar degenerative disease
- posterior lumbar interbody fusion (PLIF)
- posterior lumbar fusion (PLF)
- hybrid surgery
- adjacent segment disaese
Introduction
Lumbar degenerative disease (LDD) is one of the most common musculoskeletal disorders and a major cause of low back pain, accounting for approximately 19% of the cases that spine surgeons will encounter in their career. With the aging population, the incidence of LDD has increased by 27% from 1997 to 2015, imposing significant economic and societal burdens on society.1 LDD mainly includes conditions such as lumbar disc herniation, spinal stenosis, and spondylolisthesis, with the main clinical symptoms being low back pain, radiating leg pain, sensory abnormalities, and intermittent claudication. For patients who have poor response to conservative treatment, surgery is the optimal choice. Surgical intervention involves removing the compressed nerves through discectomy and stabilizing the lumbar spine through interbody fusion, which fundamentally improves the symptoms of LDD.2
Since its first description by Cloward in 1953, posterior lumbar interbody fusion (PLIF)3 has been increasingly used in the treatment of LDD. Other fusion surgeries commonly used include posterior lumbar fusion (PLF), transforaminal lumbar interbody fusion, and anterior lumbar interbody fusion4 However, PLIF and PLF remain the mainstream approaches as classic posterior approaches and are widely used in clinical practice.5 Numerous studies have confirmed that both PLIF and PLF have their advantages and disadvantages.6,7 However, the inconsistent results of these studies make it difficult to reach a consensus on which fusion method is superior. This is especially challenging when dealing with multilevel LDD and selecting an ideal long-segment fusion technique. The primary focus of most lumbar fusion surgeries is to reduce the occurrence of surgery-related complications, particularly adjacent segment degeneration (ASDeg) and adjacent segment disease (ASDis).8,9
Therefore, we attempted to combine PLIF and PLF as a novel hybrid fusion surgical approach. In this technique, PLIF surgery is performed on the most severe segment, while PLF surgery is used for the remaining segments. We hypothesized that this approach could overcome the limitations mentioned earlier, reduce the occurrence of ASDeg, and improve fusion rates. Thus, the aim of this study was to compare the clinical outcomes and radiological results of PLIF, PLF, and hybrid surgery (PLIF + PLF) in the treatment of LDD.
Methods
Patient Selection
This study was a single-center, retrospective cohort study. The study was approved by the Ethics Review Committee of the Sixth Medical Center of the Chinese People’s Liberation Army General Hospital (Ethics Approval Number: HZKY-PJ-2021-18). After establishing the inclusion and exclusion criteria (Table 1), a total of 408 patients underwent 1 of 3 types of surgical approaches either with PLF, PLIF, or hybrid surgery based on the patient’s clinical symptoms, radiological evidence, and the surgeon’s experience between January 2014 and September 2022. Clinical and radiological data were collected from all patients at preoperative, 3-, 6-, 12-, 36-, and 60-month follow-up time points.
The inclusion and exclusion criteria used in this study.
Surgical Technique
All surgeries were performed by the same surgical team consisting of a senior spine surgeon (D.R.) and 2 assistant spine surgeons (Q.H. and C.W.). The surgeries were conducted under general anesthesia, and all patients were placed in a prone position. A posterior midline incision was made to expose the spinous processes, laminae, and transverse processes. In general, all patients underwent posterior decompression, including laminectomy, foraminotomy, and nerve root decompression. All patients underwent pedicle screw fixation. Bone graft material was harvested from the lamina and spinous processes for posterior lateral fusion, and interbody fusion devices were used to fill and fuse the intervertebral space.
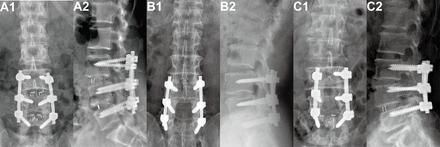
In the PLIF group, the lower one-third of the proximal adjacent lamina was removed and the medial aspect of the facet joint was resected. After the complete removal of the intervertebral disc, a trial cage was sequentially inserted into the disc space to restore the normal intervertebral disc height. Autogenous bone harvested from the lamina and spinous processes was placed inside the cage and interspace, followed by the insertion of an appropriate cage into the disc space. In the PLF group, autogenous bone was placed between the denuded transverse processes and facet joints on both sides. In the hybrid surgery group, usually the severe degenerative segment was given PLIF fusion approach, while the PLF approach was applied to the mild degenerative segment. Figure 1 illustrates the radiographic images of these 3 surgical techniques.
Radiographic images of 2-level lumbar fusion. (A1 and A2) Postoperative anteroposterior and lateral x-ray images of posterior lumbar interbody fusion (PLIF). (B1 and B2) Postoperative anteroposterior and lateral x-ray images of posterior lumbar fusion (PLF). (C1 and C2) Postoperative anteroposterior and lateral x-ray images of hybrid surgery combining distal PLIF with proximal PLF.
Postoperative Rehabilitation
After surgery, patients underwent rehabilitation exercises under the guidance of a physician. Patients were encouraged to perform lower limb functional exercises while in bed. After the removal of the drainage tube, patients were instructed to wear a lumbar support brace and gradually resume walking activities. Generally, patients were discharged around 1 week to 10 days postoperatively and were educated on how to perform rehabilitation exercises for functional recovery.
Clinical Outcome Assessment
The visual analog scale for pain, Japanese Orthopedic Association (JOA) score (for lumbar spine), Oswestry Disability Index (ODI), and 36-item Short Form Health Survey (SF-36) were used to evaluate the improvement of patients’ lower limb pain, back pain, neurological function, disability, and quality of life after surgery. These evaluations showed significant improvements in these indicators postoperatively. Furthermore, surgical time, estimated blood loss during surgery, intraoperative and postoperative complications, and drainage volume at 3 days postoperatively were compared among the groups. Adverse events included sensory deficiency, screw loosening, cage subsidence, cage retropulsion, wound infection, rod breakage, screw breakage, pseudarthrosis, and others.
Complications related to the surgery were also recorded. We focused on ASDeg and ASDis for the present study. ASDeg was assessed using the criteria reported by Min et al.10 The diagnostic criteria for ASDeg include the presence of at least 1 of the following radiological findings: (1) >10% reduction in intervertebral disc space; (2) anterior or posterior translation of the vertebral body >4 mm; (3) >10° change in the flexion-extension angle of the lumbar spine; (4) asymptomatic disc protrusion or spinal canal stenosis; (5) hypertrophy or proliferation of the facet joints; (6) formation of osteophytes >3 mm; (7) lumbar scoliosis; (8) compression fractures of the lumbar vertebrae. ASDis is defined as the occurrence of neurological symptoms in adjacent segments with degenerative changes. We recorded the overall incidence of ASDeg and ASDis among the groups, as well as the incidence of ASDeg in the upper and lower segments within each group. For fusion at the L5 to S1 level, the lower adjacent segment was not included in the statistical analysis. The displacement of the fusion device was evaluated using lateral lumbar spine x-rays, including assessing cage subsidence and cage retropulsion. Cage subsidence is defined as a displacement of the cage exceeding 2 mm beyond the endplate of the adjacent vertebral body.11 Cage retropulsion is defined as a displacement of the cage posteriorly beyond the vertebral wall or into the spinal canal.12,13
Radiographic Evaluation
At each follow-up time point, lateral lumbar spine x-ray images were used to evaluate the fusion status and lumbar lordotic angle (LL). The Cobb method was employed to measure the LL, which is the angle formed by a line parallel to the endplate of the L1 vertebral body and another line parallel to the endplate of the S1 vertebral body.
Two experienced radiologists (A.X. and L.T.), who were blinded to the clinical data and outcomes, evaluated the fusion status using anteroposterior and lateral x-ray images of the lumbosacral region. In case of disagreement between the 2 radiologists regarding fusion, additional lumbar spine computed tomography scans were performed to confirm the fusion status. The criteria for fusion after PLF were the presence of visible bony trabeculae crossing the intertransverse space, indicating osseous bridging observed on x-ray images. Fusion after PLIF was defined as continuous bony trabeculae passing through the interface between the cage and the vertebral endplates on both sides. Hybrid fusion was defined when both PLIF and PLF criteria for fusion were met. On flexion-extension x-ray images, the intervertebral motion between the fused segments was less than 4° in all groups, with no evidence of screw loosening or rod breakage.14,15 Complete fusion, incomplete fusion, nonfusion, or pseudoarthrosis formation was categorized based on whether all fused segments achieved fusion.16
Statistical Analysis
Normality tests were performed on all continuous variables. If the data followed a normal distribution, they were presented as mean ± SD. Otherwise, they were presented as median (interquartile range). Categorical data were presented as percentages (%). The χ 2 test or Fisher’s exact test was used to compare the differences in proportions between groups. One-way analysis of variance (ANOVA) was used to compare the differences in clinical scores and radiographic parameters among groups. For repeated measurements, repeated measures ANOVA was employed. In case of severe skewness of the data, the Kruskal-Wallis H test was used instead of ANOVA to explore the differences in data distribution. A P value < 0.05 was considered statistically significant. All data processing and analyses were conducted using SPSS 26.0.
Results
Patient Characteristics
A total of 408 patients were included initially; with 30 patients were lost to follow-up (7.4% dropout rate), and 378 patients were included in the final analyses. Among them, 122 patients were in the PLIF group, 126 in the PLF group, and 130 in the hybrid group. In the PLIF group, there were 122 patients (61 men) with a mean age of 58 ± 9 years. There were 46 cases of lumbar disc herniation, 61 cases of lumbar spinal stenosis, and 15 cases of degenerative lumbar spondylolisthesis. The number of surgical levels was 3 segments in 70 patients and 4 segments in 52 patients. In the PLF group, there were 126 cases (42 men) with a mean age of 60 ± 8 years. Among them, there were 47 cases of lumbar disc herniation, 45 cases of lumbar spinal stenosis, and 34 cases of degenerative lumbar spondylolisthesis. The number of surgical levels was 3 segments in 78 patients and 4 segments in 48 patients. In the hybrid group, there were 130 cases (39 men) with a mean age of 59 ± 10 years. Among them, there were 33 cases of lumbar disc herniation, 61 cases of lumbar spinal stenosis, and 37 cases of degenerative lumbar spondylolisthesis. The number of surgical levels was 3 segments in 78 patients and 4 segments in 52 patients. There were no statistically significant differences among the 3 groups in terms of age, gender, diagnosis, number of surgical levels, comorbidities, or mean follow-up time. The demographic characteristics of patients in each group are shown in Table 2.
Patient characteristics between 3 groups.
Surgical-Associated Outcomes
The average surgical time in the PLIF group was 221 minutes, which was significantly longer than the PLF group (170 minutes) and the hybrid group (197 minutes; F = 25, P < 0.001). The average estimated blood loss in the PLIF group was 375 mL, which was significantly higher than in the other 2 groups (F = 16, P < 0.001). Similarly, the postoperative drainage volume in the PLIF group was also significantly higher (435 vs 366 mL and 390 mL; F = 8, P < 0.001).
Clinical Outcomes
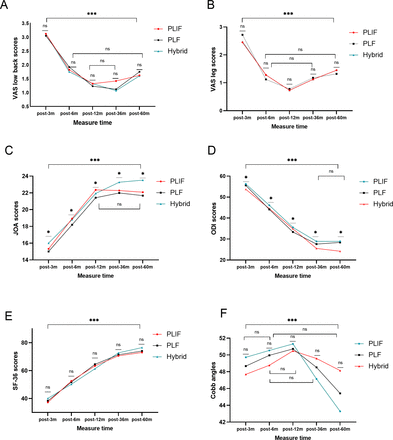
Repeated measures ANOVA showed that visual analog scale scores for the lumbar spine and leg improved to varying degrees as the follow-up time increased (all P measure time < 0.001), but there were no significant differences in scores among the groups (all P measure time * group > 0.05). With increasing follow-up time, the JOA score significantly improved (Fmeasure time = 389; P measure time < 0.001), but there was also a slight statistical difference among the groups. Specifically, at different follow-up points, the hybrid group showed a slight improvement in JOA scores compared with the PLF group (F measure time * group = 2.167; P measure time * group = 0.047). Similarly, with longer patient recovery time, ODI scores improved significantly (F measure time=939; P measure time < 0.001), and the improvement was more pronounced in the hybrid group (F measure time * group = 2.826; P measure time * group = 0.006). It is worth noting that the measurement time and group interaction analysis of SF-36 scores showed significant differences (F measure time * group = 3.589; P measure time * group < 0.001), but pairwise comparisons between the groups did not detect any statistical differences. Therefore, this difference is attributed to the effect of measurement time points (F measure time= 1,020; P measure time < 0.001). Table 3 and Figure 2 provide specific parameters and trends at each measurement point.
Changes in patient-reported scores and radiographic parameters at different follow-up time points (3, 6, 12, 36, and 60 months) after surgery. Abbreviations: JOA = Japanese Orthopedic Association; ODI = Oswestry Disability Index; PLF = posterior lumbar fusion; PLIF = posterior lumbar interbody fusion; SF-36 = 36-item Short Form Health Survey; VAS = visual analog scale.
Repeated-measures ANOVA for comparison of clinical outcomes at each follow-up time.
Radiographic Parameters
As the follow-up time increased, all 3 groups showed significant improvement in postoperative Cobb angle for LL (F measure time = 18.176; P measure time < 0.001). Although there was a significant difference in the interaction analysis between measurement time points and groups (F measure time * group = 3.594; P measure time * group < 0.001), pairwise comparisons between the groups did not show any statistically significant differences. Therefore, the difference is attributed to the effect of measurement time points. Consequently, there were no significant statistical differences in LL angles among the 3 groups (Table 3 and Figure 2). Additionally, the overall fusion rates in the hybrid group (93%) and PLIF group (91%) were significantly higher than the fusion rate in the PLF group (84%; P = 0.031).
Complications
The intraoperative complication rate in the PLIF group was 15%, significantly higher than the hybrid group (9.8%) and the PLF group (6.6%; P = 0.05; Table 4). The postoperative adverse event rate in the PLIF group (24.4%) was significantly higher than the PLF group (16.4%) and the hybrid group (12.5%; P = 0.022).
Intraoperative and postoperative complications between 3 groups.
5-Year ASDeg and ASDis
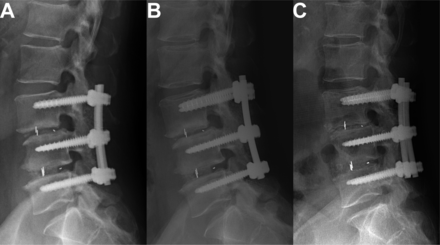
In the hybrid group, the occurrence rate of ASDeg for multilevel fusion was 29%, which was significantly lower than that in the PLIF group (42%) and the PLF group (37%; P = 0.044). The overall occurrence rates of 5-year ASDis for multilevel fusion in the PLIF, PLF, and hybrid groups were 12.3%, 9.9%, and 7.6%, respectively, with no statistically significant differences (P = 0.338; Table 4). A total of 8 patients (2.2%) underwent revision surgery, including extension of fusion segments, removal of original fixation and implementation of new adjacent segment fusion, new vertebral plate resection and decompression surgery, and percutaneous endoscopic discectomy. Other patients obtained symptom relief through conservative treatment. Figure 3 shows the imaging and management measures for ASDis in the 3 groups. In the PLIF and hybrid groups, cage subsidence occurred in 8 (6.9%) and 4 cases (2.8%), respectively (P = 0.117), and cage migration posteriorly occurred in 5 cases (4.4%) and 2 cases (1.4%), respectively (P = 0.179). In the PLIF group, revision surgery was performed to remove and reposition the displaced cage in 4 cases with accompanying neural compression symptoms, while the remaining 3 cases without neural compression symptoms received conservative treatment. In the hybrid group, 1 patient underwent revision surgery due to posterior protrusion of the cage with associated neurological symptoms. Figure 4 shows the cases of cage migration and subsidence in the PLIF and hybrid groups.
Imaging of adjacent segment degeneration development in hybrid surgery 54 months postoperatively. (A and B) Anteroposterior and lateral x-ray images of hybrid surgery at L3–L5. (C–E) Computed tomography images showing disc protrusion and spinal stenosis at the adjacent levels L5–S1.
Imaging of posterior cage migration and subsidence. (A) Lateral x-ray of a patient showing proper cage position at L3/4, 3 months after surgery. (B) Mild subsidence of the L3/4 cage observed at 36 months postoperatively. (C) Significant subsidence of the L3/4 cage observed at 60 months postoperatively.
Discussion
PLF and PLIF, as classical posterior lumbar techniques, each have their advantages and disadvantages in theory and practice. Nevertheless, the absolute superiority of PLF or PLIF has not been established. Furthermore, the complexity of determining the optimal surgical approach and the controversy over its efficacy increase with the number of fusion segments, particularly in patients with different pathological conditions.
For multilevel degenerative lumbar disease, a simple PLIF procedure may not be suitable for relatively mild degenerative segments and can increase the risk of postoperative ASDeg.17 Conversely, using PLF alone to treat severe multilevel degenerative lumbar disease may not be sufficient and could increase the risk of nonfusion and had more complications.18 Therefore, hybrid surgery combining PLIF and PLF techniques might address the limitations of using PLIF or PLF alone in the management of multilevel degenerative lumbar disease. Hybrid surgery provided more targeted treatment to improve patient symptoms and function during long-term follow-up in this study.
PLF, PLIF, and hybrid techniques used in this study were able to improve pain and reduce functional impairment in all patients. The results of the ODI showed no significant difference in baseline ODI scores among the 3 groups, and all patients experienced improvement, which was sustained for up to 60 months. From 36 months postoperatively onward, the improvement trend in ODI scores gradually diminished, and at the last follow-up, the hybrid group showed a significant improvement in ODI scores. It is undeniable that immediate postoperative functional score improvement was mainly due to adequate decompression of the neural element. However, maintaining lumbar stability and a lower incidence of complications contributed to the improvement at 36-month follow-up. With the prolonged duration follow-up, natural aging and complications (especially ASD and hardware failure) might lead to the development of new symptoms or recurrent symptoms, thus exacerbating the trend of symptom deterioration. The hybrid group maintained favorable clinical and radiological outcomes during long-term follow-up, which could be attributed to its lower complication rate compared with the PLIF group.
Restoration and maintenance of LL have been emphasized and are considered key indicators for evaluating sagittal balance and the severity of degeneration in the spine.19 This study compared the pre- and postoperative measurements of LL among the 3 groups. The results showed that all groups experienced significant improvement at 3 months postoperatively, and this improvement was correlated with clinical outcomes. The improvement in LL was better in the PLF and hybrid groups compared with the PLIF group. Our findings were consistent with the studies conducted by Farrokhi et al6 and Korovessis et al,20 which demonstrated that the improvement in LL was greater in the PLF group than in the PLIF group. Additionally, the study by Kuraishi et al21 showed that the improvement in LL in the PLIF group was not superior to the PLF group. However, some studies have reported conflicting results, with Kim et al22 and Müslüman et al23 suggesting that PLIF better restores proper spinal alignment compared with PLF. Based on the current research, it is speculated that achieving good LL values during PLIF surgery requires higher technical demands, and its stiffness may not adapt well to the compensatory mechanism of postoperative pelvic retroversion to maintain sagittal plane balance.24 Another possible explanation is the lack of comprehensive assessment of sagittal balance in the surgical planning process. Surgeons often bend titanium rods to a specific curvature based on their individual experience before installation, which may result in inadequate LL correction, particularly in the technically demanding PLIF procedure. Therefore, adequate preparation and assessment of sagittal plane balance are necessary in the preoperative phase to determine the optimal LL angle required for achieving sagittal balance for degenerative lumbar disease.
In this study, the addition of interbody cages significantly improved interbody fusion rates compared with the PLF group. Our findings are consistent with most studies that suggest higher fusion rates with PLIF compared with PLF.6,25 However, there are also conflicting results in some studies. A meta-analysis conducted by Liu et al26 showed comparable fusion rates between PLIF and PLF for the treatment of degenerative spondylolisthesis. Based on Wolff’s law,6 it can be inferred that a greater compressive fusion bed, as achieved with interbody fusion, is closer to the physiological load-bearing anterior spine. In our study, the hybrid surgery group also had higher fusion rates compared with the PLF group. One possible explanation is that combining distal interbody fusion provides a stable base support and promotes bone healing (interbody fusion). Another possibility that cannot be ruled out is the lower accuracy of x-ray radiographs used to assess fusion definitively.
ASDeg and ASDis have become the most concerning and debated complications following lumbar fusion surgery. From 1998 to 2014, the use of lumbar fusion with internal fixation increased dramatically by 262%.4,27 The occurrence rate of ASDeg after PLF surgery varies from 5.2% to 49% in various studies.17,28 In our study, the overall occurrence rates of ASDeg were consistent with these findings, with a rate of 42% in the PLIF group, 29% in the PLF group, and 37% in the hybrid group. The cumulative revision rates at 2 years postlumbar fusion surgery were 12.5%, increasing to 19.3% at 4 years, with ASDis being the main cause for revision.29 ASDis following lumbar fusion surgery significantly affects patient prognosis and quality of life and is receiving increasing attention from spine surgeons. However, the causes and mechanisms of its occurrence remain controversial, primarily focusing on biomechanical changes after lumbar fusion or the natural process of intervertebral disc degeneration.30–32 Numerous risk factors contribute to the development of ASDeg after lumbar fusion surgery, but different scholars have reached different conclusions through survey studies, sometimes even opposing conclusions.33–35
In the present study, there were no statistically significant differences in baseline data among the 3 groups, which increased the comparability of the study. Additionally, by selecting adjacent disc degeneration levels that were similar, we avoided the influence of potential preoperative risk factors. Our study results showed that the occurrence rate of ASD increased gradually with longer follow-up, but the hybrid group had a lower rate of ASDeg compared with the PLF and PLIF groups (P = 0.044).
The decrease in lumbar spine elastic modulus and the increased stiffness caused by long-segment fusion make it difficult for adjacent segments to adapt to biomechanical changes. These biomechanical changes include stress concentration and increased intervertebral disc pressure, making the adjacent segments more susceptible to degeneration. In addition to the length of the fused segment, our study results also showed that, under the same fusion length, the occurrence rate of ASDeg was significantly higher in the PLIF group compared with the PLF and hybrid groups. Apart from the differences in mechanisms and scope between PLIF and PLF, during the PLIF procedure, more extensive disruption of the posterior column structures is required for the safe insertion of interbody fusion cages. This may be a contributing factor to the increased risk of ASDeg. Similar to our findings, Ma et al36 conducted a study on risk factors for ASDeg and found that long-segment decompression with interbody fusion was 1 of the risk factors for postoperative ASDeg, as an increased extent of decompression and fused segments means more resection of the lamina and posterior ligament complex, leading to biomechanical changes in the spine and a higher likelihood of ASDeg. These conclusions have also been supported by biomechanical experiments37 and a long-term randomized controlled trial.38
In addition to patient- and surgery-related factors, the correlation between the sagittal balance of the lumbar spine and ASDeg has received increasing attention in recent years. Due to the lack of full-length standing spine x-ray images, we only measured LL to assess the impact of sagittal balance on ASDeg. Wang and Ding33 reported a correlation between postoperative LL angle and the occurrence of ASDeg through a meta-analysis. Nakashima et al39 also reached the same conclusion, suggesting that restoring appropriate LL plays a crucial role in preventing ASD in PLIF. In our study, the PLF and hybrid groups showed better improvement in LL compared with the PLIF group, resulting in a lower occurrence rate of ASDeg. Oda et al40 found that loss of LL after surgery can lead to excessive movement in the lumbar spine, thereby increasing biomechanical stress on adjacent segments. It is hypothesized that restoring the LL angle can reduce the occurrence of postoperative ASDeg. However, it is imperative to conduct more comprehensive and rigorous studies with larger cohorts to elucidate the underlying mechanisms between LL and adjacent segment degeneration. In the context of Dynesys, a dynamic stabilization system, a meta-analysis by Wang et al.41 suggested that its use might mitigate the incidence of ASD. Nevertheless, the literature on the protective efficacy of Dynesys against ASD is marked by inconsistencies.42–44 To date, there is a paucity of long-term studies that definitively demonstrate whether dynamic stabilization can effectively reduce the prevalence of ASD.45
Cage displacement is 1 of the complications that can occur after PLIF, including anterior displacement, posterior migration, and subsidence. However, anterior cage migration is relatively rare. In this study, we only compared the occurrence of posterior migration and subsidence between the PLIF and hybrid groups because these 2 types of displacement can lead to severe consequences, such as direct compression of nerve roots or cauda equina, pseudoarthrosis, or failure of internal fixation. In our study, the incidence rate of cage subsidence in the PLIF group was 6.9%, which is consistent with previous studies (0.8%–25%).46–48 The rate was substantially higher than that in the hypbrid group (2.8%) but was not statistically significant. This difference may be attributed to the higher occurrence rate of cage subsidence in the PLIF group due to a larger number of fused segments compared with the hybrid group. However, the present sample size of this study did not result in statistically significant differences. This finding aligned with the view proposed by Kimura et al13 that multilevel fusion is 1 of the risk factors for cage displacement. Therefore, we believed that hybrid surgery might reduce the occurrence rate of cage displacement (subsidence and posterior migration) after PLIF for multisegment degenerative diseases. Although there was no statistically significant difference between the PLIF and hybrid groups in cage displacement, this complication still requires careful attention in clinical practice and further research in the future.
Our study also had certain limitations. First, it was a retrospective study. Some cases were excluded due to incomplete data, and the inclusion of 3 types of patients might be considered selection bias. Second, the incidence rate of posterior cage migration was relatively low, and the number of cases included in this study was limited, making it difficult to draw certain convincing conclusions.
Conclusions
All 3 surgical techniques might effectively improve the clinical symptoms of patients with degenerative lumbar disease effectively. The hybrid technique demonstrated comparable efficacy to PLIF and PLF in increasing fusion rate, reducing complications, and decreasing the occurrence of ASDeg in multilevel fusion cases significantly.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
Ethics approval This study was approved by the Institutional Review Board of the Sixth Medical Center of PLA General Hospital (Former Navy General Hospital; HZKY-PJ-2023-1).
Author’s Contributions Zhenbiao Zhu, Dike Ruan, and Anwu Xuan: conception/design, data analysis, interpretation, and manuscript writing. Qing He and Dike Ruan: surgical management and provision of study material or patients. Zhenbiao Zhu, Anwu Xuan, and Cheng Xu: data collection and analysis and drafting the manuscript. Zhenbiao Zhu, Anwu Xuan, and Liang Tang: data measurement, data analysis, interpretation, and manuscript writing. All authors have read, revised, and approved the submitted manuscript.
Data Availability The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2024 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.