Abstract
Introduction Autograft has been the “gold standard” for orthopedic bone grafting applications, but with some clinical challenges. Here we present the rationale and clinical outcomes supporting the use of a bone substitute material that consists of a mixture of two calcium phosphates (HA and ß-TCP), which are integrated into a silicon xerogel matrix, promoting nanocrystalline apatite layers on the surface of the material following implantation into a physiological environment.
Methods Twenty-four patients with a median age of 53.80 (36–81) years underwent lumbar spinal fusion for degenerative disease, selected by clinical presentation, X-rays, and MRI findings. Subjects were evaluated preoperatively and postoperatively at 1, 3, 6, and 12 months. The outcome assessment consisted of visual analog scale (VAS), Oswestry Disability Index (ODI), and radiological assessment analyzing the state of fusion on X-ray and CT evaluation by 3 independent radiologists.
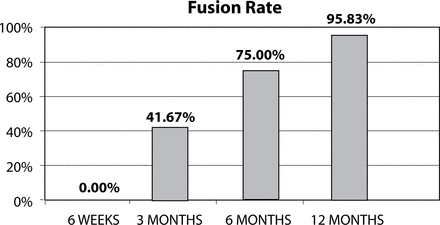
Results All patients completed 12-month follow-up. The mean VAS decreased from 9.3 (± 0.9) to 2.4 (± 1.6) and the mean ODI decreased from 55.0 (± 9.2) to 19.3 (± 11.4) at 12-month follow-up. Three months after surgery, 10 patients (41.67%) had solid fusion based on analysis of CT scans and dynamic radiographs. At 6 months postoperatively, the fusion rate had increased to 75% (18 patients). Twelve months after surgery, 95.83% of patients had solid fusion (23 patients).
Conclusions The clinical results from this study of silicon matrix calcium phosphate are consistent with previous in vitro studies indicating that this material stimulates formation of a bioactive layer and provides an effective bone graft material for lumbar fusion applications. In comparison with previous studies involving rhBMP-2, silicon matrix calcium phosphate provided a lower fusion rate at 3- and 6-month follow-up points, but after 12 months, the fusion rate was similar, with no statistical differences and lower overall costs. No clinically relevant adverse events were associated with either the cage or graft material. With increasing evidence of high rates of enhanced fusion development in this spinal application, additional research is encouraged, including longer periods of followup, to further confirm the efficacy of silicon matrix calcium phosphate as a safe and effective bone graft substitute.
INTRODUCTION AND BACKGROUND
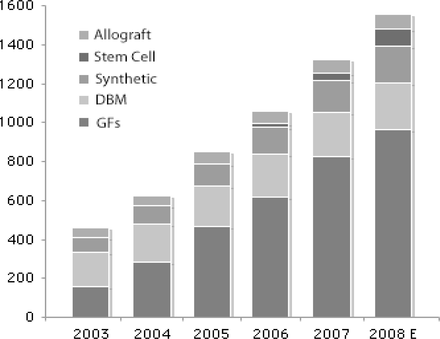
It is estimated that in the US alone nearly 600,000 surgical procedures employed some form of bone grafting material in 2007 (Table 1). In the spinal products industry, bone substitute products represented a market value of around $1.3 billion in 2007 and are expected to reach $1.6 billion this year (Figure 1). More than 75% of all procedures use some form of bone graft material—whether allograft, autologous or synthetic (Table 1).
Annual US sales of bone substitute products ($ in millions).
Source: PearlDiver Technologies Data and Consulting Services
Spinal Surgical Procedures Employing Bone Grafts 2007
The broader applications of bone grafting include long bone surgery (metaphyseal defect, diaphyseal segmental defect, total joint reconstruction) as well as anterior or posterior spinal fusion.
Autograft has traditionally been the “gold standard” for orthopedic bone grafting applications. The clinical challenges specific to spinal fusion include (1) need for graft harvest, (2) a non-union rate between 5% and 35%, (3) graft failure, and (4) a limited supply of autograft for multilevel fusion.1–3 In addition there is donor site morbidity of up to 25–30%, including hematoma, pain, increased operation time, blood loss, host metabolic hindrances, previous failed fusion, and the necessity to exclude smokers, diabetics, and osteoporotic patients, etc.
A common alternative to autograft is the use of allograft, ie, bone harvested from cadavers or intraoperatively harvested excess bone (eg, femoral heads from total hip arthroplasty). Such bone must be processed to remove infection agents and cellular material which may cause complications and graft rejections. While the use of allograft materials is common and results generally in good patient outcomes, it is expensive and only available in limited quantities. Consequently there has been a need for synthetic bone graft materials, such as ceramics. Such materials are employed as osteoconductive scaffolds and generally in conjunction with autogenous components (eg, blood, bone marrow, etc.), which supply the necessary cells and proteins for osteoinduction and osteogenesis (Table 2).4
Properties of Bone Graft Materials
In response to this trend, and primarily due to the high morbidity after iliac crest graft harvesting,1, 3 synthetic and natural sources of bone graft substitute materials have been under investigation for over 30 years. Current research in spine surgery is focusing on use of resorbable bone implants. There have been many studies on the formation of bone substitute composites, as bone is a natural composite primarily composed of inorganic calcium and phosphates and organic fibrous collagen, and controlled by a variety of hormones, cytokines, and growth factors. Bone regeneration requires a suitable scaffold for the growth of bone cells and a viable, well vascularized host bed. Previous studies have shown an optimal pore range for the scaffold of 200-300 μm, with the average human osteon size being approximately 223 μm. However, porosity level can also adversely affect the mechanical properties of the scaffold.1, 3, 5, 6 Calcium phosphates, such as hydroxyapatite (HA), promoted for its chemical and crystallographic similarities to natural bone mineral, have received special attention, particularly due to their osteoconductivity.7
The development of porous hydroxyapatite and related calcium phosphate ceramics appear to be an effective direction given that calcium phosphates offer the possibility of promoting the growth of bone tissue (Table 3).8
Main Calcium Phosphate Compounds
In the development of new materials as bone substitutes, the basic requirement of these materials are biocompatibility with good osteoconduction, and sufficient load bearing mechanical properties for their specific applications. The primary requirements for the overall composite, including any complementary graft material used, are to be osteoconductive, osteogenic, and biodegradable.5, 9
The bioactivity of hydroxyapatite has been demonstrated to be strongly dependent on a number of both physical and chemical factors. Previous studies have demonstrated that substitution of ionic species, such as carbonate and fluoride, enhances the bioactivity of HA.7, 10 Therefore, in this study, we present the use of an advanced bone substitute material, silicon matrix calcium phosphate, that consists of a mixture of the 2 calcium phosphates, hydroxyapatite (HA) and ß-stricalciumphosphate (ß-TCP), integrated into a silicon dioxide xerogel matrix. Silicon dioxide (Si O2) in a hydrated form results in promotion of nanocrystalline apatite layers on the surface of the material following implantation into a physiological environment.
Especially important are the physical structures in the nanorange of the material, which allow for a very large internal surface of approximately 90 m2/g. Mixed with autologous blood, this structure guarantees a rapid spongelike distribution of the blood cells throughout the material (Table 2).
This study determined the preliminary clinical and radiological results of silicon matrix calcium phosphate, a calcium phosphate/silicon dioxide xerogel combination, and its rate of osseous integration in spinal fusion procedures.6, 9
METHODS
This prospective, longitudinal, clinical and radiological study evaluated the outcome of spinal fusion procedures using silicon matrix calcium phosphates as a bone substitute, in a group of 24 patients undergoing anterior spinal fusion for symptomatic degenerative diseases in the lumbar spine. Patients admitted to the study had lumbar degenerative disc disease, as evidenced by back pain of discogenic origin, with degeneration of the disc confirmed by radiologic studies (decreased disc height, endplate sclerosis, osteophytes, black disc disease) and/or provocative discography. An economic evaluation was done to compare the global costs between surgeries using silicon matrix calcium phosphate and surgeries using INFUSE Bone Graft (Medtronic, Minneapolis, Minnesota), including hospitalizations, medical devices, implantable materials, outpatient and emergency room visits, drugs, laboratory tests, and diagnostic imaging tests.
The Oswestry Disability Index (ODI)11 survey was used for subjective evaluation of functional outcome, and visual analogue scales (VAS) were recorded to establish pain severity at various pre- and postoperative intervals
Plain roentgenograms, including anteroposterior, lateral, and flexion and extension views, as well as computed tomography (CT) scans of the spine, were used to evaluate the evolution of the fusion in this cohort of patients. The evaluation was performed by 3 blinded radiologists determining establishment of fusion, defined as less than 5° of angular motion and an absence of radiolucent lines over at least 50% of the implant surfaces. CT scans were similarly evaluated by 3 blinded neuroradiologists for evidence of fusion, which in this case was defined by the presence of continuous trabecular bone growth through both of the cages.12 Data were analyzed using paired Student's t test, with P values below .05 being considered statistically significant.
RESULTS
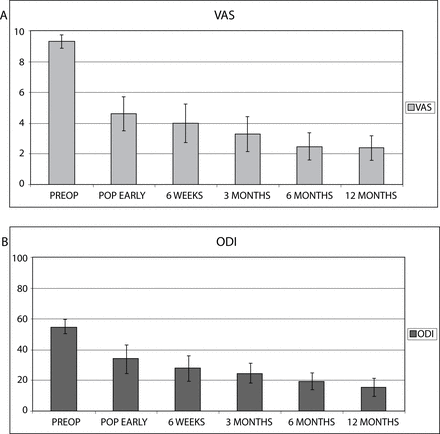
In 2005 and 2006, a total of 24 patients underwent fusion surgery using silicon matrix calcium phosphate, with addition of bone marrow aspirate from the lumbar vertebral body, as a bone graft. All patients underwent an anterior lumbar interbody fusion (ALIF) procedure using a KLA PEEK Cage (Scient'x, Maitland, Florida). The material was mixed with bone marrow and placed in the cage. The mean age was 53.80 ± 14.7 years (range: 36–81) with a gender distribution of 15 females (62.5%) and 9 males (37.5%). The mean hospital stay was 1.8 (± 0.5) days, and mean blood loss was 86 cc (± 35). All patients completed 12-month follow-up visits. Twelve months following surgery, mean VAS pain scores decreased from 9.3 (± 0.9) to 2.4 (± 1.6), and mean ODI scores decreased from 55.0 (± 9.2) to 19.3 (± 11.4) (Figure 2).
(A) Visual analogue scale (VAS) average values from preoperative (PREOP), postoperative early (POP EARLY), 6-week, 3-month, 6-month, and 12-month follow-up points. (B) Oswestry Disability Index (ODI) average values from preoperative (PREOP), postoperative early (POP EARLY), 6-week, 3-month, 6-month, and 12-month follow-up. STD-standard deviation.
A comparison between global costs was done to analyze the general spending involved in both surgical treatments. The significant difference between both grafts was in the upfront cost (Table 4). Silicon matrix calcium phosphate is 53% cheaper than INFUSE, with similar pre- and postoperative spending and similar fusion success rates.
Comparison of Global Surgery Costs
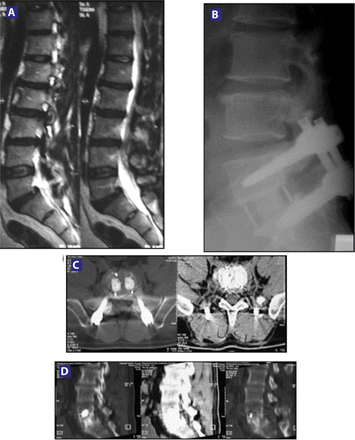
Based on analysis of CT scans and conventional lateral flexion-extension radiographs, at the 3-month follow-up point, 10 patients (62.5%) were found to be fused. Six months after surgery, 75% of patients (18 patients) treated with silicon matrix calcium phosphate were found to be fused (Figure 3), which was increased to 95.83% (23 patients) at 12 months (Figure 4). Patients were not considered to be fused unless all 3 independent radiologists agreed upon this finding. Only 1 patient was not fused 12 months after surgery, but presented bone bridging and remodeling of the endplate (Figure 5). There was no evidence of clinically significant (> 1 mm) subsidence or any anteroposterior migration or rotation of the grafts (Figure 6).
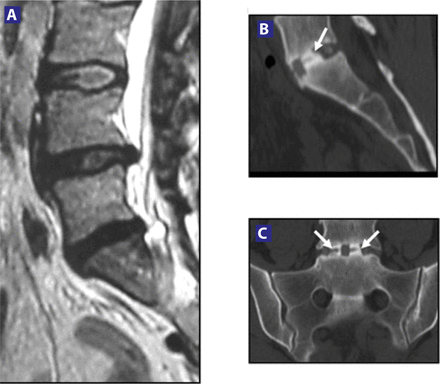
(A) Preoperative MRI showing Degenerative Disc Disease (DDD) at L5-S1, confirmed by discography. (B) Six-month follow-up: evidence of fusion in a lateral X-ray. (C) & (D) Six-month axial and sagittal CT scan showing fusion.
Fusion rates from 6-week, 3-month, 6-month, and 12-month follow-up time points.
(A) Preoperative MRI showing DDD at L5-S1, confirmed by discography. (B) Twelve-month sagittal CT scan showing bridging bone. (C) Twelve-month coronal CT scan confirming bridging bone. This patient had not been considered fused at 12-month follow-up. Note: the unlinked trabecular bones (white arrows).
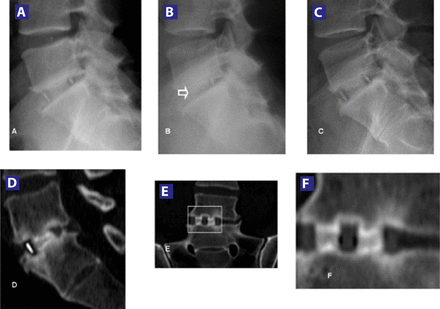
(A) X-ray views at 1 month postoperatively. (B) 3 months postoperatively, evidence of the fusion developing (arrow). (C) Demonstrated solid fusion on X-rays and CT scan from the 6-month postoperative time point. (D) CT sagittal view at 6-month follow-up. (E) Coronal view showing solid fusion 6 months after surgery. (F) Zoom to confirm the fusion.
DISCUSSION
The favorable clinical results of this study are consistent with previous in vitro13, 14 observations supporting a beneficial influence of silicium ion incorporation in calcium phosphate graft combinations. These early clinical and radiological results support the evidence of effectiveness of the materials’ biochemical and physical properties, and their ability to profoundly enhance the processes of osteoconduction. The results of earlier in vitro and preclinical studies of these materials6, 15 indicate that the acceleration in bone apposition may partly result from an up-regulation in osteoblast cell metabolism.16, 17 An excellent mean fusion rate of 95.83% at 12-month follow-up, as well as favorable clinical outcomes, was obtained in this group of patients. In addition, the cheaper surgical cost in comparison to INFUSE18, 19 encourages the use of silicon matrix calcium phosphate. Longer periods of follow-up are required for establishing mid- and long-term clinical results.2, 20–22
CONCLUSIONS
The clinical results from this study of silicon matrix calcium phosphate are consistent with previous in vitro13, 14 studies indicating that this material stimulates formation of a bioactive layer and provides an effective bone graft material for lumbar fusion applications.23, 24 In comparison with previous studies involving rhBMP-212, 25 silicon matrix calcium phosphate provided a lower fusion rate at 3- and 6-month follow-up points, but after 12 months, the fusion rate was similar, with no statistical differences and a cheaper cost. No clinically relevant adverse events were associated with either the cage or graft material. With increasing evidence of high rates of enhanced fusion development in this spinal application, additional research is encouraged, including longer periods of followup, to further confirm the efficacy of silicon matrix calcium phosphate as a safe and effective bone graft substitute.
Footnotes
No funds were received to support this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Institutional Review Board approval was obtained from Santa Rita Hospital, São Paulo, SP, Brazil
- Received November 7, 2007.
- Accepted February 29, 2008.
- Copyright SAS - Spine Arthroplasty Society 2008
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License, permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.