Abstract
Minimally invasive spinal surgery has made tremendous progress. The retroperitoneal lateral lumbar interbody fusion and oblique lumbar interbody fusion techniques are increasingly used to treat a variety of lumbar spinal conditions. As with other minimally invasive techniques, the theoretical advantages include reduced blood loss, improved postoperative pain due to less retraction and smaller incisions, faster recovery, and eliminating the need for an approach surgeon. It allows the placement of a large cage that spans the apophyses and enables the achievement of indirect decompression as well as coronal and sagittal deformity correction. As experience grows and the techniques evolve further, indications might be expanded beyond their current limitations. However, as these techniques continue to grow in popularity, evidence-based risk-stratification systems are required. This study reviews the literature about the development and application of lateral lumbar interbody fusion and oblique lumbar interbody fusion, with a discussion of its outcomes, approach-related complications specific to the lateral technique, and areas of new research.
Introduction
One of the most notable developments in lumbar spine surgery over the past few decades has been the advent of minimally invasive approaches to the anterior lumbar spine.
The retroperitoneal lateral lumbar interbody fusion (LLIF) technique is increasingly used to treat a variety of lumbar spinal conditions. The lateral transpsoas technique was first described by McAfee et al in 1998 and later elaborated on by Ozgur et al in 2006.1,2 In 2012, Silvestre et al reported a new minimally invasive variant that takes advantage of the window between the peritoneum and the psoas muscle, in lieu of splitting the muscle, and called it oblique lumbar interbody fusion (OLIF) or the anterior-to-psoas (ATP) approach.3 OLIF uses the anatomical space between the aorta/inferior vena cava and the psoas muscle to access the disc space.
Both LLIF and OLIF take a lateral approach around the psoas to access the lumbar spine. As with other minimally invasive techniques, the theoretical advantages include reduced blood loss, improved postoperative pain due to less retraction and smaller incisions, faster recovery, and elimination of the need for an approach surgeon.4 The prominent advantage of LLIF and OLIF should be indirect decompression, which means a resolution of nerve compromise with an increase in disc height. However, an appropriate selection of patients is crucial to resolve symptoms without direct decompression.
The present article reviews the literature about the development and application of LLIF and OLIF with a discussion of its outcomes, approach-related complications specific to the lateral technique, and areas of new research.
Data Collection Methods
A literature search was performed on PubMed and Web of Science using the keywords: LLIF, OLIF, ATP approach, direct lateral interbody fusion, and extreme lateral interbody fusion (XLIF). All results from June 2014 through June 2024 were first evaluated by reading the titles and abstracts. If the article met the criterion of studying the above procedures, it was included in the review.
We excluded articles that were outdated and did not use clear methods. Our primary sources for trials were high-quality randomized controlled trials, systematic reviews, and meta-analyses. Out of the 173 searched literature reports, the abstracts of 169 articles were reviewed, 47 of which were excluded after reviewing the abstract. The remaining 122 articles were reviewed, and another 48 articles were excluded after quality evaluation. In summary, 74 articles were reviewed. Of note, potential author bias due to personal experiences and preferences, which may affect the reproducibility, may be the main limitation of the current review.
History and Evolution of Lumbar Interbody Fusion
Over time, fusion techniques have evolved, and lumbar interbody fusion (LIF), which involves inserting a cage and a bone graft into the intervertebral space, became popular as a procedure that offers both stability and fusion.5,6 Early LIF procedures include posterior lumbar interbody fusion (PLIF) by Cloward in 1943, anterior lumbar interbody fusion (ALIF) by Lane and Moore in 1948, and transforaminal lumbar interbody fusion (TLIF) by Harms and Rolinger in 1982.7–9
In 2001, Pimenta first reported using a tubular distractor to approach spinal fusion through the retroperitoneal space and the psoas major muscle.10 OLIF was reported by Silvestre et al in 2012.3 Compared with LLIF, the OLIF approach uses the anatomical space near the psoas major muscle without cutting it off. It can not only effectively avoid the risk of vascular injury caused by anterior surgery but also avoid injury to the lumbar plexus nerve caused by damaging the psoas major muscle during LLIF. In addition, neuromonitoring is not necessary during the operation, and the incidences of hip flexion weakness and thigh numbness are lower than with LLIF, so it has attracted much attention from surgeons.
In an anatomical and imaging study of the feasibility of the OLIF surgical approach, Davis et al studied the anatomical structure of the L2 to S1 lateral surgical pathway in 20 cadaveric specimens (11 men and 9 women). They placed each specimen in a lateral position to measure the width of the surgical window and the L2 to S1 intervertebral space. In the static state, the average widths of the operation windows were 18.6 mm (L2–L3), 19.25 mm (L3–L4), and 15.0 mm (L4–L5). When the psoas major muscle was stretched lightly, its average width was 25.5 mm (L2–L3), 27.05 mm (L3–L4), and 24.45 mm (L4–L5).11
Uribe et al divided the lumbar vertebrae into 4 zones between the anterior and posterior edges of the vertebral body in the sagittal position. In the lateral decubitus position, the lumbar plexus is distributed on the dorsal side of zones IV and III (L1–L2 and L3–L4) and at the intersection of zones II and III (L4–L5).12 The cage is inserted into the posterior 1/3 position of the intervertebral space, increasing the height of the disc space and the foramen, which is the principle of indirect decompression of the spinal canal.
Indications/Contraindications for LLIF and OLIF
The indications for lateral lumbar approaches include degenerative scoliosis, spondylolisthesis, instability, infection, lumbar revision, and moderate spinal stenosis. Two of the most popular indications for these are indirect decompression of the neural elements by restoring a collapsed disc space and the reduction of spondylolisthesis or scoliosis. The placement of a large interbody cage has been shown in several studies to significantly increase the foraminal cross-sectional area and central canal cross-sectional area and improve the correction of deformity.13–16 The LLIF and OLIF procedures can be used in revision surgery when an open decompression has a relatively high complication rate. As experience with the lateral lumbar approach has increased, publications have shown that the lateral approach can be effectively used to treat infectious, neoplastic, and traumatic thoracolumbar conditions.
In patients with osseous stenosis, congenital stenosis, or intraspinal space-occupying lesions such as tumors, tightening the ligament is not effective. In addition, indirect decompression will not relieve symptoms in patients with severe spinal stenosis. Similarly, LLIF and OLIF are not recommended for patients with spontaneous fusion of the intervertebral space or posterior facet joints. According to the minimally invasive spinal deformity surgery classification proposed by Mummaneni et al, type III adult deformity requires osteotomy and 3-column and thoracic spine fusion, which is not suitable for OLIF.17 Radiation distributed along the approach path, retroperitoneal infection/abscess, and a history of surgery are contraindications to the lateral approach.18 The psoas size and position and vascular anatomy can make the approach to L4/5 difficult, and these structures should be scrutinized on cross-sectional imaging as part of the surgery planning process.
OLIF Techniques
OLIF, an anterior-to-psoas approach to interbody fusion, was first adopted by Meyer in 1997, and the term was coined by Silvestre et al in 2012. Subsequently, Woods et al further developed and popularized the technique.19 This approach offers minimally invasive access to the disc space via the corridor between the psoas and the vessels and is suitable for fusing levels L2 to L5.11 In addition, Woods et al developed the concept of OLIF L5 to S1, which is an anterior approach performed in a lateral decubitus position when the L5 to S1 region needs to be accessed.19
Procedure for OLIF L2 to L5
For OLIF L2 to L5, the patient is positioned in a right lateral decubitus position. Once positioned, the legs are slightly flexed, and a line is drawn across the disc level.19 The fascia of the external oblique muscle is first incised, followed by gentle blunt dissection of the external oblique, the internal oblique, and the transversalis muscles. After working on the transversalis fascia, the retroperitoneal fat plane is reached, followed by anterior retraction of the peritoneal sac and posterior retraction of the anterior belly of the psoas muscle. After the psoas muscle is retracted, the disc space is visualized, and a guide wire is inserted, followed by a series of dilators. Subsequently, a retractor is positioned over the dilators and can be anchored to the vertebral body using a pin. After disc removal and sequential trialing, a contralateral annular release is performed using a blunt-tipped shaver or Cobb elevator. Sequential trials dilate the disc space and allow indirect decompression. Finally, an appropriately sized cage is inserted in an end-to-end fashion to prevent further subsidence (Figure 1).
(a) The patient is positioned in a right lateral decubitus position, and “favorable oblique” position is achieved, which means a comfortable approach angle for surgeons. (b) A retractor is positioned over the dilators and can be anchored to the vertebral body using a pin. (c) After disc removal and sequential trialing, a contralateral annular release is performed using a blunt-tipped shaver or Cobb elevator.
Procedure for OLIF L5 to S1
A line is drawn across the L5 to S1 disc level, and a second line is drawn from the center of the L5 to S1 disc.19 Then, approximately 2 finger breadths anterior to the anterior superior iliac spine, a third line is drawn connecting the first and second lines, and the incision is made along the third line. Dissection is made as described in the OLIF L2 to L5 procedure. The common iliac artery pulse can be felt on the anterior border of the psoas, and the common iliac vein is medial to the artery. After successfully releasing the adventitial layer, the left common iliac vein can be gently retracted laterally, if needed. Discectomy and interbody cage placement are performed in a manner similar to ALIF (Figure 2).
(a) A line is drawn across the L5 to S1 disc level. Subsequently, a second line is drawn from the center of the L5 to S1 disc. Finally, approximately 2 finger breadths anterior to the anterior superior iliac spine, a third line is drawn connecting the first and second lines where the incision is made. (b) Discectomy and interbody cage placement are performed in a manner similar to anterior lumbar interbody fusion.
Advantages of OLIF Over LLIF
Several factors make OLIF more convenient than LLIF. First, the surgical oblique approach enables direct visualization of the disc space, ureters, major blood vessels, and most of the psoas muscle. It also allows visualization of the anterior disc margin and, thereby, better anterior placement of the cages.20 The main advantage of OLIF over LLIF is that no dissection of the psoas is required.3,21,22 Furthermore, OLIF can reduce the use of EMG neuromonitoring during the procedure, although some patients will still experience hip flexion weakness due to prolonged psoas retraction. Nonetheless, because OLIF preserves most of the psoas muscle fibers, it is less associated with hip flexion weakness, with only 1.2% to 13.9% of patients experiencing postoperative hip flexion weakness, compared with 4.9% to 31.4% of patients receiving LLIF.23–31 Because the lumbar plexus is also avoided, OLIF carries a reduced likelihood of lower limb sensory and motor weakness, compared with LLIF. Various studies attribute better postoperative visual analog scale and Oswestry Disability Index scores following OLIF to the reduced incidence of psoas muscle injury.29
Clinical Applications of Lateral Lumbar Approaches
A common indication for LLIF and OLIF is adult lumbar spondylolisthesis with lumbar spinal stenosis. Traditionally, TLIF was the surgical method most commonly used for that indication because it can directly decompress and reduce lumbar spondylolisthesis with pedicle screws. However, direct decompression-related complications were worrisome. LLIF and OLIF can fully open the disc space for indirect decompression. The height of the intervertebral foramen and the area of the spinal canal increase significantly after cage insertion. In addition, coronal and sagittal imbalances can be effectively corrected with a larger cage. The use of LLIF and OLIF with an angular fusion cage can effectively restore lumbar lordosis and maintain the sagittal balance of the lumbar spine. It can also increase the contact area of the bone graft, and its immediate stable support provides a good environment for bone fusion.
Fang et al reported 20 patients with lumbar spondylolisthesis treated with OLIF and found that the degree of lumbar spondylolisthesis was well recovered.32 The height of the intervertebral space increased from 6 ± 3.6 mm preoperatively to 10.8 ± 1.7 mm, and lumbar lordosis increased from 39.2 ± 8.4 to 45.0 ± 7.8. Magnetic resonance imaging showed that the size of the intervertebral foramen and the area of the dural sac increased from 78.1 ± 31.2 mm2 and 73.4 ± 29.3 mm2 before the operation to 141.7 ± 29.5 mm2 and 124.5 ± 26.6 mm2 after the operation, respectively. The data show that OLIF is effective in the early stage of lumbar spondylolisthesis with secondary spinal stenosis, with minimal trauma and accurate reduction of vertebral slippage. Liu et al compared PLIF to OLIF based on the concentration of C-reactive protein and creatine kinase in serum.33 The OLIF group experienced less influence on the internal environment of the body after the operation than the PLIF group. The perioperative indicators of average incision length, intraoperative bleeding volume, and postoperative hospital stay have been compared between OLIF and PLIF, and the advantages of OLIF were significant because PLIF requires extensive dissection of the multifidus muscle, laminectomy, and facet process, resulting in spinal instability and scar adhesion.
Degenerative Conditions
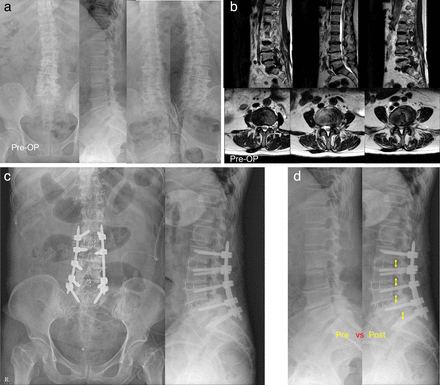
Recent literature shows good results from using OLIF to treat degenerative disc disease, degenerative spondylolisthesis, and adjacent segment degeneration.34–38 The treatment of spondylolisthesis is a common indication for LLIF and OLIF. Studies have shown improvement in clinical outcomes and partial to complete reduction in both grade I and grade II disease.35,36,39 Sato et al reported indirect decompression in spondylolisthesis and improvements in the cross-sectional area of the spinal canal.22 However, longer-term studies are needed to show the maintenance of those improvements (Figure 3).
(a) Plain radiographs of a 70-year-old woman with multilevel lumbar spinal stenosis. (b) Preoperative magnetic resonance images show multilevel moderate-degree stenosis with bilateral foraminal narrowing. (c) Plain radiographs show increased disc height after multilevel oblique lumbar interbody fusion cage insertion. (d) Postoperative plain radiograph shows percutaneous screw fixation without direct decompression with relief of symptoms.
Adult Lumbar Deformity
Adult degenerative scoliosis describes new-onset scoliosis after skeleton maturity, and it is associated with a longer disease course and more complications than juvenile scoliosis. The purpose of adult deformity surgery is the reconstruction of the alignment and decompression of the nerve. LLIF and OLIF are widely accepted for such treatment, including opening the intervertebral space, indirect decompression, and bone graft fusion, with secondary posterior internal fixation. Traditional deformity procedures are large operations with significant blood loss, approach-related muscle trauma, and high complication rates of up to 30%.40 In addition to the powerful deformity correction associated with the use of the minimally invasive LLIF and OLIF technique, it avoids the risks of the large traditional posterior deformity procedures.41 Many studies have demonstrated significant deformity correction and improvement of long-term outcomes from using the LLIF technique to treat adult spinal deformity.42,43 Uribe et al compared the complications of minimally invasive LLIF with those following hybrid and open procedures for adult spinal deformity and reported substantially less blood loss and a lower complication rate, with similar improvements in outcomes.44 Sharma et al followed up with43 patients with scoliosis who underwent OLIF and reported that the coronal Cobb angle was corrected to 3.75 on average.45 Anand et al suggested that the coronal Cobb angle could be corrected from 22 to 7 after an OLIF operation.46 Ohtori et al performed OLIF on 12 patients with scoliosis.47 The imaging data revealed that the sagittal vertical axis decreased from 140 mm to 27 mm, pelvic tilting (PT) decreased from 37 to 23, pelvic incidence-lumbar lordosis decreased from 41 to 8, the coronal Cobb angle decreased from 42 to 5, and lumbar lordosis increased from 6 to 37. A potential disadvantage of using LLIF techniques alone to treat adult spinal deformity is their limited ability to achieve significant sagittal plane correction. However, the use of LLIF in combination with open posterior techniques has been shown to improve sagittal plane correction to levels comparable to traditional posterior correction, with a lower complication rate.48–50
Recently, the anterior column realignment procedure has been described as a lateral interbody fusion technique with a greater capacity for sagittal deformity correction. In managing deformity with significant sagittal plane involvement, anterior longitudinal ligament (ALL) release has been performed to enhance sagittal correction. ALL release has shown sagittal plane correction even greater than that of pedicle subtraction osteotomy with substantially less surgical time and blood loss.51,52
Adjacent Segment Disease and Revision
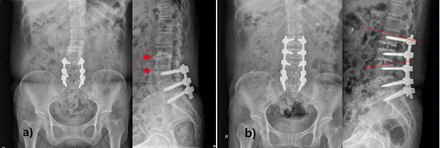
Adjacent segment disease (ASD) is the development of symptomatic degeneration of the spine adjacent to a previously fused segment. Lateral surgery for the treatment of ASD is emerging because it can reduce the morbidity of a revision posterior approach. By using a retroperitoneal approach, OLIF can prevent adhesions, shorten the operation time, decrease trauma to the body, and improve the patient’s tolerance to surgery. In addition, ASD has been hypothesized to be exacerbated by the disruption of posterior ligamentous structures, so reducing the violation of the posterior part could be beneficial. A retrospective series of patients with ASD treated with LLIF reported that patients treated with either standalone LLIF or LLIF with circumferential fusion showed improvement in their clinical and radiographic outcomes.38 Zhu et al followed up 17 patients treated with OLIF and 19 patients treated with PLIF.53 In the OLIF group, the operation time, intraoperative bleeding volume, time bedridden, and hospitalization time were all significantly shorter than in the PLIF group (Figure 4).
(a) Plain radiographs of 63-year-old woman who was operated at L4-L5-S1 6 years ago show adjacent segment degeneration at L2-L3-L4 level. (b) Postoperative plain radiographs show restoration of disc height and lumbar lordosis with oblique lumbar interbody fusion surgery.
Infection, Trauma, and Neoplastic Disease
Patients with trauma, infection, or metastatic disease are often not medically optimized for surgery, making minimally invasive surgical approaches particularly appealing. Many of these conditions have historically required extensive posterior approaches, and avoiding approach-related morbidity is a strong incentive to use a different approach. Studies report the treatment of infection, trauma, and neoplastic disease with LLIF and OLIF. Often patients with diskitis/osteomyelitis have comorbid conditions that increase the risk of complications from an open posterior technique. The literature contains a few reports about using the lateral approach for debridement and stabilization. Those studies demonstrated effective eradication of infection and no failures of debridement during the follow-up periods.54–56 Some researchers have reported tumor resection and stabilization through the lateral technique.57–59 Adoption of the lateral approach avoids the more extensive midline posterior incision, which can reduce the risk of wound complications and allow for a shorter delay to the initiation of chemotherapy and radiation, if appropriate. In treating thoracolumbar trauma, the lateral approach can be used to perform anterior decompression and place an interbody cage. In such situations, LLIF and OLIF provide direct neural element decompression and anterior stabilization that allows for sagittal alignment correction. Supplemental lateral plating or posterior percutaneous pedicle screw placement creates a biomechanically stronger construct than posterior pedicle screw placement alone.60 Hong et al demonstrated the effective treatment of thoracolumbar trauma through the lateral approach with and without posterior instrumentation.61
Complications
Vascular Injury
A reduced rate of vascular injury is one of the advantages of OLIF, compared with ALIF. However, vascular injury has still been reported in both LLIF and OLIF. Abdominal vascular injury is the most serious intraoperative complication of OLIF. Arterial injury rates in OLIF were previously reported to be 0.3% to 2.4%, including injury to major blood vessels and segmental vessels.41,62–64 The meta-analysis by Walker et al compared the rates of vascular injury in OLIF and LLIF and found a reported rate of 1.8% for major vessel injury with the OLIF approach, compared with 0.4% with the LLIF technique (P < 0.05).30 The local anatomical relationships among tissues and organs should be clearly understood. The abdominal aorta is located on the left anterolateral side of the lumbar spine, and the vena cava is located on the right anterolateral side. Molinares et al suggested that a gap of less than 1 cm between the psoas muscle and the anterior vertebral artery is not suitable for this approach.65 In addition, when breaking through the contralateral annulus fibrosus, the breakthrough point should not be too close to the front of the vertebral body where the inferior vena cava is located because it is easy to rupture and difficult to repair.
Vascular injuries have been reported to occur more frequently with L5 to S1 levels in OLIF.66 Tannoury et al used the left and right pre-psoas approaches to access L5 to S1 without assistance from an access surgeon, reporting a 0.3% incidence of minor vascular injuries and no major vascular injuries in their series.67 Woods et al used the left intra-bifurcation approach in their exposure to L5 to S1 with the assistance of an access surgeon; a vascular injury rate of 2.9%, which increased to 4.3% when L5 to S1 was included, was reported (P < 0.05).19 Chung et al reported major vascular injuries in 11.5% (3/26) and 5.1% (2/39) of the patients who underwent OLIF and ALIF, respectively.68 They also retrospectively reviewed the relationship between the left common iliac vein and the L5 to S1 disc, discovering that vascular injuries were more likely to occur when the left CIV is located over the medial two-thirds. Surgeons have to remember the incidence of vascular injury during the L5 to S1 OLIF procedures, and access surgeons should be ready for risky cases.
Thigh Symptoms
Thigh symptoms such as anterior thigh pain, dysesthesias, numbness, or hip flexor weakness are quite common from the lateral approach, and to an extent, they are an expected postoperative finding in most cases. The reported rates in the literature vary from 10% to 60%.69–74 However, most such symptoms are limited and resolve within a few weeks. LLIF has been associated with a 20% to 40% rate of thigh numbness or pain associated with prolonged muscle retraction.75 For that reason, surgeons are still advised to minimize retractor time during this procedure. A recent systematic review and meta-analysis identified 63 articles describing 6714 patients who underwent 11,325 levels of lumbar fusion (average 1.69 levels per patient).76 Neurological deficit was the most common complication, with a pooled transient postoperative deficit rate of 36% in an aggregate sample of 5046 patients. A small series of 18 patients in whom LLIF was used to treat spondylolisthesis reported that a third (33%) of patients developed a sensory deficit of the thigh but also that all cases had resolved within 6 months postoperatively.77 In contrast to those high rates, a more recent, single-institution study of single-level LLIF found that only 2.6% (6 of 230 patients) suffered severe thigh weakness (defined as strength of 3/5 or lower in either hip flexion or knee extension) of more than 6 weeks in duration, and 22 patients (9.6%) sustained sensory loss consistent with the surgery.78 A possible explanation for that lower complication rate might be an improvement in the techniques over time, with newer retractors potentially associated with a reduced rate of LLIF-related thigh symptoms.79,80 Other proposed advances also show promise in decreasing the incidence of these complications through the direct visualization of the nerves. A small retrospective series comparing LLIF with OLIF found that OLIF was associated with a lower risk of thigh numbness.81 Likewise, a similar comparison of OLIF and LLIF in 43 total patients found OLIF to be significantly superior in preventing nerve deficits of the thigh (P < 0.05).82 However, the heterogeneity of the patient populations between the 2 groups in those studies makes it difficult to draw a solid conclusion.
Perhaps the most well-recognized risk of the lateral approach to lumbar spine surgery is injury to the lumbosacral plexus. Such injury can occur in the abdominal wall, retroperitoneal space, or at the level of the psoas.83 The nerve most commonly reported to be injured is the lateral femoral cutaneous nerve, which is most at risk at the L4/5 level. A recent study demonstrated an approximately 3.7% incidence of plexus pathology from the lateral approach, but the rate of persistent deficit after 18 months fell to 2.3%, illustrating the potential for recovery over time.84 A recent meta-analysis exploring complications of LLIF found that the transpsoas technique was associated with more frequent neurologic and thigh symptoms than the ATP technique.30
Lastly, although intraoperative neuromonitoring of motor-evoked potentials has been found to be effective in preventing postoperative lumbar plexus deficits during LLIF, its routine use is controversial.85,86 An impetus for the development of the oblique approach was to reduce this complication by avoiding the psoas and subsequent traction injury to the nerves.2
Pseudohernia
Pseudohernia is an abdominal wall bulge without violation of the abdominal wall. In the setting of operative trauma, the etiology is related to the denervation of abdominal wall muscles. The pseudohernia rate reported in the literature is 1.8%; however, much of the literature on this topic fails to distinguish pseudohernia from incisional hernias, limiting the ability to clarify the rate of this complication.87 In a series reported by Dakwar et al, most cases of pseudohernia were resolved by 6 months postoperatively. To prevent any hernia-related complications, minimizing both the skin incision and directional splitting of abdominal muscle by using a table-mount retractor might be beneficial.87
Urethral Injury
Urethral injury is an intraoperative complication with a lower incidence than in other surgeries. Various authors agree that the level with the greatest risk for a ureter lesion is L2 to L3.88 The ureter is located behind the peritoneum and descends vertically into the pelvis. Extraperitoneal fat is pushed to the ventral side. The operation must not be conducted through fat, which would risk injury to the ureter. The possibility of a ureter lesion should be considered in cases of abdominal pain, fever, leukocytosis, or abdominal distention. Kubota et al demonstrated that delayed contrast-enhanced computed tomography (CT) and retrograde urography are useful in diagnosing such an injury. Urethral injury can be avoided by complete retraction of the retroperitoneal fatty tissue before starting the discectomy and the anterior mobilization of the ureter.89,90
Uncommon Complications
Other complications, such as visceral injuries or intraoperative durotomies, are very uncommon and generally reported in the 1% or below range.28,91 Sometimes contralateral nerve root damage is encountered. Surgeons should be cautious when preparing the contralateral side endplate with Cobbs and understand that insertion of too long a cage can induce root irritation on the far lateral side. The incidence of sympathetic chain injury varies, ranging from 1.7% to 8.7%.92–95 Gragnaniello et al demonstrated that the sympathetic trunk has to be mobilized by smooth retractor blades; even sacrifice produces only warming of the affected leg that is unnoticed by patients. Only a small percentage of patients experienced ileus or infection.92
Challenges of OLIF
Radiation Exposure
Repeated fluoroscopy is needed to prepare the inter-vertebral discs, so patients receive more radiation than they do for posterior lumbar fusion. Striano et al reported 134 interbody fusions; 80 were performed with a posterior approach (TLIF/PLIF), 43 via an anterior approach (ALIF) with posterior pedicle fixation, and 9 were performed with a lateral approach (LLIF/XLIF).96 Mean radiation dose per case was 136.4 mGy (SE 17.3) for ALIF, 108.6 mGy (16.9) for LLIF/XLIF, and 60.5 mGy (7.4) for TLIF/PLIF. They identified lateral approaches, increased body mass index, minimally invasive techniques, and more caudal operative levels as significantly associated with increased radiation exposure. They identified several novel drivers of radiation exposure during interbody fusion procedures, including the relative importance of technique and the level at which the fusion is performed. More caudal levels of intervention and lateral-based techniques had significantly greater radiation exposure. To minimize radiation exposure, physicians should notice harmful effects of radiation, and further protection is needed.
Incomplete Decompression
An additional drawback of LLIF and OLIF is that the decompression of the neurological elements risks incomplete relief. In a retrospective series of 28 patients undergoing OLIF at 53 levels, patients were evaluated with intraoperative CT myelograms after cage placement, and in 9 patients, (11 levels) the CT revealed inadequate radiographic decompression of the nerve root. All 9 of these patients then underwent additional direct posterior decompression.97 There is interest in defining risk factors for successful indirect decompression through LLIF. A prospective multicenter study evaluated several radiographic variables for their ability to predict the failure of indirect decompression during LLIF, as defined by the need for revision surgery or inadequate improvement of symptoms at 6 months.98 Of them, only bony lateral recess stenosis was found to be a significant predictor of poor indirect decompression.99 Park et al reported that neither the radiographic appearance of the facet joints or facet angulation nor CVA pre- or postoperatively showed a relationship with clinical outcomes.100 In that study, the mild-to-moderate stenosis group showed favorable outcomes with indirect decompression for up to 1 year, and those authors emphasized the importance of cage position in the posterior part of the disc, which might maximize the foraminal height increase.
Cage Subsidence
Despite the use of wide interbody cages during LLIF and OLIF procedures, cage subsidence can still occur. The incidence of sedimentation ranges from 2.9% to 10%.19 Several factors can account for cage subsidence, which is related to the technique, the implant material, and the bone quality of the patient.101–103 Factors in maintaining the stability of the cage include bone quality and the integrity of the endplate, proper size, elasticity force of the anterior and posterior longitudinal ligaments, and the tensile stress of the posterior ligament complex. The endplate can easily be damaged during preparation for the operation, causing iatrogenic subsidence, especially with poor bone quality. It is important to bear in mind that the endplate is concave, making it resistant peripherally and weaker centrally; therefore, only the edge of the cage can support the endplates immediately after implantation. Liu et al observed 67 patients who underwent OLIF. In 18 cases with posterior fixation, no cage sedimentation occurred during follow-up.104 In the cases with unilateral fixation and standalone fixation, the incidences of cage sedimentation were 3.85% and 26.09%, respectively. Obviously, posterior fixation decreases the incidence of sedimentation, but the increased cost, prolonged operation time, and greater damage to the body should be taken into consideration. The subsidence risk can be effectively reduced by considering the pre-existing bone health, conducting careful patient selection, evaluating patient medical status, and practicing meticulous intraoperative techniques, such as avoiding aggressive endplate preparation.105,106
Advanced Considerations
Standalone LLIF and OLIF
The anterior and posterior techniques for interbody fusion of the lumbar spine sacrifice stabilizing ligamentous and bony structures, which then often necessitate supplemental fixation. The lateral interbody fusion technique preserves the ALL, posterior longitudinal ligament, and posterior elements. As a result of those preserved stabilizers, surgeons have expanded the indications for standalone LLIF. For example, standalone LLIF has been hypothesized to be sufficient for the treatment of ASD when dynamic instability is not present.107 A recent study of standalone LLIF showed clinical and radiographic improvement for 18 months of postoperative follow-up with only a 12% reoperation rate for the addition of posterior instrumentation.108 Biomechanical studies of standalone LLIF show stability that compares favorably with other interbody fusions with adjunctive posterior fixation.109,110 Ultimately, the decision to proceed with standalone LLIF should be made on a case-by-case basis because it does carry some risk of cage subsidence, compared with lateral constructs with adjunctive fixation. Healthy, thin, nonsmoking patients with good bone quality and 1- or 2-level pathology can be considered for standalone LLIF if a large footprint interbody is placed, and the underlying pathology does not involve significant instability.111,112
Staged Surgery
Masuda et al reported 293 consecutive ASD patients (107 in the 2-staged group and 186 in the 1-day group) who underwent corrective surgery using LLIF between 2012 and 2021.113 In this cohort, 19 (18.4%) patients in the 2-staged group and 43 (23.1%) patients in the 1-day group experienced any systemic perioperative complication within 30 days following ASD surgery. In the propensity score (PS)-weighted cohort, compared with the patients undergoing 1-day surgery, no association with the risk of systemic perioperative complications was seen in patients undergoing 2-staged surgery (PS-weighted OR 0.78, 95% CI 0.37–1.63; P = 0.51). Our study suggested that 2-staged surgery was not associated with risk for perioperative systemic complications following ASD surgery using LLIF. Yamato et al reported that 138 patients of mean age 69.8 (range, 50–84) years who met the study inclusion criteria were included.114 The 2-stage group (n = 75) underwent a staged anterior-posterior surgical procedure, and the control group (n = 63) underwent single-stage surgery. There was no significant between-group difference in the incidence of perioperative complications, except for deep wound infection (reoperation is necessary for surgical site infection). Revision surgery within 3 months of the initial surgery was more common in the control group (n = 8, 12.7%) than in the 2-stage group (n = 3, 4.0%). Spinopelvic parameters and patient-reported outcome measures were significantly better in the 2-stage group at 2 years postoperatively. The complication rate for planned 2-stage surgery was similar to that of previous posterior-only single-stage surgery. However, early reoperation was less common, and the degree of spinal correction and clinical results were significantly better after 2-stage surgery.
OLIF Recent Advances and Future
Single-Position Surgery
Despite the significant, demonstrated benefits of OLIF, sometimes repositioning the patient with additional stabilization is needed. One-stage surgery requires the patient to be moved from the lateral decubitus position to prone. During that process, re-draping and repositioning the patient prolongs the surgical duration and might not be suitable for patients with contraindications.115,116 Moreover, it is known that prolonged surgery increases the risk of surgical site infections.117,118 However, with the advent of single-position surgery (SPS), both XLIF and OLIF can be performed in a single position, predominantly the former, along with posterior stabilization.119–122 There are currently 2 main approaches to SPS: lateral-SPS and prone-SPS, in which the patient is placed in either the lateral decubitus or prone position, respectively. Both approaches are reported to significantly decrease surgical times, with reductions of 60 minutes to up to 135 minutes, ultimately leading to a decrease in the duration of hospitalization.115,122–126 However, surgeons can lack familiarity with performing posterior stabilization in the lateral position. Basic tasks, such as laminectomy for posterior decompression and the insertion of pedicle screws, become challenging, ultimately limiting the size of the posterior construct. Furthermore, the lateral position offers limited lordosis correction, compared with that that can be accomplished in a prone position. These drawbacks have resulted in greater incidences of facet joint violation and pedicle screw breach.127
Prone positioning allows for superior restoration of lordosis and safer corridors for LLIF and facilitates posterior instrumentation and access.128 In a recent cadaver study, the femoral nerve was reported to be located more posteriorly at L4 to L5 in the prone position compared with lateral.129 They reported a larger safe zone at L4 to L5, and it may decrease the incidence of neurologic complications associated with LLIF. Prone positioning also indirectly increases operating room efficiency because it is a familiar position for both surgeons and staff. On the other hand, proponents of lateral positioning argue that the lateral position allows for a simultaneous anterior approach, and the prone position heightens the potential morbidity of a vascular injury. However, to date, there are extremely few studies directly comparing radiographic or clinical outcomes between prone LLIF and lateral LLIF and no study comparing single-position minimally invasive surgery deformity correction in the prone vs lateral position.130
Navigation-Assisted
CT-based navigation techniques are known to reduce radiation exposure and allow for safe implant positioning. Minimally invasive techniques are typically fluoroscopy dependent due to the lack of direct visualization of patient anatomy. Surgeons have described using CT-based navigation to perform LLIF procedures.131,132 Benefits of navigation for LLIF include precise incision localization, reduced incision length, and minimized soft-tissue disturbance. Navigation could also reduce the risk of vascular or visceral complications, although studies investigating that possibility have yet to be published.
Robot-Assisted
The use of robots in spine surgery is evolving from the use of navigation. The adoption of preoperative planning software and robotic guidance for pedicle screw placement enhances the ability to adhere to and execute a surgical plan with the utmost accuracy.133 Huntsman et al reported early results from a series of 55 cases, where lateral-SPS was performed by a single surgeon, who started with pedicle screw placement under robotic guidance, continued with LLIF, and, finally, fixed rods to complete the construct.134 Of the 328 screws inserted, 2% were repositioned at the surgeon’s discretion, resulting in a success rate of 98% for navigated robot-assisted pedicle screw placement. Diaz-Aguilar et al published a series of cases in which 2 surgeons conducted simultaneous robotic-single position surgery, while the patient was in the lateral position. This approach effectively reduced the overall operating time, with 1 surgeon concentrating on the LLIF procedure, while the other focused on the robotic screw placement.135 Despite the small number of studies investigating the financial effects of incorporating robotic technology into these procedures, the available evidence indicates its cost-effectiveness.136 With ongoing developments and the increased availability of robotic technology, this procedure has the potential to become the standard of care in the future.
Conclusion
Minimally invasive spinal surgery has made tremendous progress. LLIF/OLIF has gained popularity as a technique for placing wide interbody devices to treat lumbar disease. It allows the placement of a large cage that spans the apophyses and enables the achievement of indirect decompression, as well as coronal and sagittal deformity correction. The advantages of LLIF/OLIF result in part from the lateral access, which allows the preservation of posterior stabilizing ligamentous structures and prevents posterior muscle damage. Currently, many surgeons choose OLIF over LLIF because the OLIF technique has shown fewer related complications than LLIF. However, surgeons should check the locations of major vessels before OLIF surgery, because the surgical corridor is located just lateral to the major vessels than LLIF. LLIF is more favorable with a high lumbar approach, and ribs may block the oblique lateral corridor. We recommend surgeons flexibly use LLIF and OLIF according to surgical level and anatomical characteristics. Minimally invasive surgery has become the main direction of spinal surgery research, and with the emergence of new technologies, LIF will be further developed. As experience grows and the technique evolves further, indications might be expanded beyond their current limitations. However, as this technique continues to grow in popularity, evidence-based risk-stratification systems are required.
Acknowledgments
This study was designed by K-MISS (Korean Minimally Invasive Spine Surgery) study group.
Footnotes
↵† Jae-Young Hong and Jaewan Soh are joint first authors.
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2025 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵