Abstract
Background Computed tomography (CT) myelography is an important imaging modality utilized in patients who cannot tolerate magnetic resonance imaging (MRI) due to severe artifact or because of the presence of MRI incompatible medical devices. In the setting of thoracic stenosis and a spinal cord stimulator (SCS), it can predispose patients to develop acute weakness and paraplegia after myelography.
Case Presentation We present 2 patients with symptomatic thoracic stenosis who developed acute paraplegia after a CT myelogram. Both patients had SCSs due to a history of back pain and were taken emergently to the operating room for SCS removal and thoracic spine decompression.
Conclusion To our knowledge, these are the first reported cases of acute paraplegia postmyelography in the presence of an SCS in the thoracic spine. This report serves as an important warning and possible emerging contraindication for ordering CT myelography in patients with thoracic stenosis in the presence of an SCS.
Introduction
Postmyelography paraplegia is a rare complication that has been underreported in the literature, with limited case reports of thoracic stenosis cited.1,2 There have not been any reported cases of acute paraplegia after computed tomography (CT) myelography in the presence of spinal cord stimulators (SCSs). Multiple theories regarding the pathophysiology of this phenomenon have been described. The most highly recognized is that the bolus of contrast injected in the intrathecal space causes an increase in the pressure gradient against the stenotic section of the spinal cord, which can lead to transient or permanent neurological deficits.1 Another theory suggests that changing cerebrospinal fluid dynamics in the thoracic cord can exacerbate the patient’s neurological deficits by compromising the spinal cord’s blood flow.1 Initial studies mentioned a 14% risk for paraplegia or paraparesis after CT myelogram if the imaging showed a complete spinal block. Nevertheless, these patients had epidural spinal cord tumors as the culprit for their spinal coning.3 Numerous authors have suggested differing possible mechanisms. Honda et al described a mobile schwannoma that compressed the spinal cord after the myelography injection.4 Other studies have cited acute paraplegia after CT myelogram, but the etiology of the weakness was found to be an expanding hematoma after lumbar puncture in patients with coagulopathies or thrombocytopenia.5–7
CT myelography is still an important diagnostic image modality in patients who are unable to undergo magnetic resonance imaging (MRI) or when severe artifact limits the quality of the image. It can play a significant role in visualizing the intradural extramedullary space and identifying cerebrospinal fluid leaks. CT myelography is an excellent tool for patients who cannot tolerate an MRI due to body habitus, claustrophobia, artifact, or the presence of an MRI-incompatible medical device.8 In the presence of previous spinal hardware, MRI produces a significant amount of artifacts. As a result, a CT myelogram is required to assess the degree of stenosis and localize the correct spinal level. Nevertheless, the presence of an SCS in patients undergoing a CT myelogram produces an additional mass effect and may lead to an exacerbation of symptoms after intrathecal injection due to its compressive effect, particularly in patients with thoracic stenosis. To our knowledge, these are the first cases reported in the literature displaying acute paraplegia after CT myelography in the presence of an SCS.
Case Presentations
Case 1
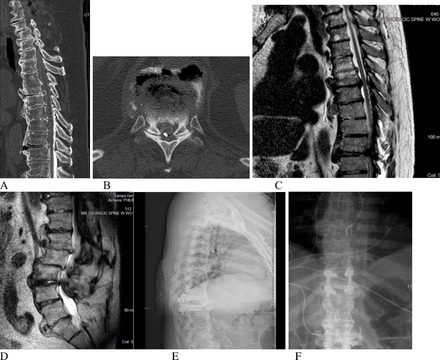
The first case is of an 80-year-old man with a medical history of hypertension and multiple spine surgeries consisting of an L4 to L5 and a C4 to C6 posterior spinal fusion. In 2019, he underwent an SCS placement that improved his chronic back pain until his arrival at our emergency department due to back pain, paresthesia of his billateral lower extremities (BLE), and loss of balance. He was ambulating with the use of a walker. On physical examination, he was 5/5 on manual muscle testing (MMT) of his BLE and 2+ throughout his deep tendon reflexes. Findings on his thoracic and lumbar MRI showed stenosis on multiple levels, including T6 to T7 and T10 to T11, and there was evidence of L3 to L4 adjacent level disease with significant motion artifact (Figure 1C–D). A CT myelogram showed a partial block at T10 to T11 and at T6 to T7 adjacent to his SCS percutaneous lead. Immediately after completion of the CT myelogram, he developed acute BLE weakness (0/5) on MMT. He was taken emergently to the operating room for a T7 to T11 laminectomy with a T10 to T11 interbody placement, L3 to L4 transforaminal lumbar interbody fusion, and SCS removal (Figure 1E–F). He had an uncomplicated postoperative course and recovered to his baseline examination of (5/5) on his BLE on postoperative day 1. He was discharged home on postoperative day 5 and completed inpatient rehabilitation.
(A) Preoperative thoracic spine computed tomography (CT) myelogram (sagittal). (B) Preoperative CT myelogram of the T11 to T12 level (axial). (C) Preoperative T2-weighted thoracic magnetic resonance imaging (MRI; sagittal). (D) Preoperative T2-weighted lumbar MRI (sagittal). (E) Postoperative thoracic x-ray images (lateral). (F) Postoperative thoracic x-ray images (anterior-posterior).
Case 2
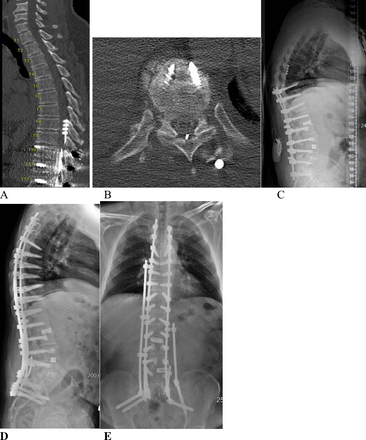
Case 2 involved a 60-year-old man with a medical history of high blood pressure and multiple lumbar spine interventions. Prior to presentation at our institution, he had undergone a T10 to S1 posterior instrumentation and fusion with multiple transforaminal lumbar interbody fusion. He had an SCS placed for back pain and failed back syndrome in the past. He presented to our hospital with BLE weakness 4/5 on MMT and BLE paresthesia when standing upright. He presented with proximal junctional kyphosis at T9 to T10 with a flat back deformity (Figure 2C). During our imaging workup, his thoracic MRI produced a significant artifact; thus, we obtained a CT myelogram to localize the focal area of stenosis. The myelogram showed a complete spinal block at T9 to T10 (Figure 2A). Immediately after the CT myelogram, he developed acute weakness of his BLE 0/5 on MMT, with complete loss of sensation below the T10 dermatomal level. He was taken emergently to the operating room for a T7 to T10 laminectomy and SCS removal with hardware extension. Then, he was staged and optimized for T4 to pelvic instrumentation and fusion with an L5 pedicle subtraction osteotomy to correct his proximal junctional kyphosis and flat back deformity (Figure 2D–E). He displayed a gradual improvement in his BLE motor strength to 4/5 and is currently ambulating with a walker after participating in inpatient rehabilitation.
(A) Preoperative thoracic computed tomography (CT) myelogram showing the myelography block at T9 to T10 (sagittal). (B) Preoperative T9 to T10 CT myelogram (axial). (C) Preoperative lateral standing scoliosis x-rays, showing previous fusion from T10 to S1 with flat back syndrome. (D) Postoperative lateral standing scoliosis x-ray images. (E) Postoperative anterior-posterior standing scoliosis x-ray images.
Discussion
These 2 cases represent the only cases, to our knowledge, of acute paraplegia after CT myelography in patients with SCS. We believe that the presence of an SCS in patients with symptomatic thoracic stenosis can be a potential contraindication for CT myelography. The combination of both the SCS and the thoracic stenosis can lead to permanent weakness after CT myelography if not diagnosed and treated emergently. As a result, physicians should be cautious when ordering this imaging modality in myelopathic patients. If myelography is deemed necessary, then the ordering physician should ensure that spine surgeons are available for immediate decompressive surgery if needed after CT. Both of our cases showed evidence of a complete “spinal block” on the myelogram (Figures 1A and 2A). A complete “myelography block” could be a predictive imaging sign that suggests a higher risk of developing acute paraplegia after a CT myelogram.
Prior cases of postmyelography paraplegia have resolved with surgical decompression via the costotransversectomy approach, similar to what we utilized to decompress the thoracic cord in both our cases.9 Some studies have suggested that postmyelography paraparesis improved with the administration of intravenous dexamethasone without the need for surgical decompression.1 This demonstrates that the pathophysiology and ideal treatment for this phenomenon are yet to be determined. We believe that all spine surgeons should be aware of this neurological entity and avoid a CT myelogram in patients with symptomatic thoracic stenosis in the presence of an SCS.
The patient in case 1 had a percutaneous lead, and the patient in case 2 had a paddle lead. This suggests that the type of SCS does not affect the risk of developing BLE weakness. Additional studies are needed to uncover the etiology of this rare entity, and the decision to obtain CT myelography should be evaluated on a case-by-case basis, particularly when it concerns patients with thoracic myelopathy and an SCS.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
IRB Approval IRB approval was obtained for the realization of this report.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2025 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.