Abstract
Objective In this article, the authors systematically evaluated the efficacy and safety of tranexamic acid (TXA) in surgeries for spinal trauma.
Methods Potentially relevant academic articles were identified from the Cochrane Library, MEDLINE, PubMed, and Google Scholar. Secondary sources were identified from the references of the included literature. RevMan software was used to analyze the pooled data.
Results A total of 7 randomized controlled trials (RCTs) and 2 non-RCTs were included in the review. There were significant differences in total blood loss (standard mean difference [SMD] = −2.54 [95% CI, −3.72, −1.37], P = 0.0001), intraoperative blood loss (SMD = −0.96 [95% CI, −1.28, −0.64], P < 0.00001), postoperative blood loss (SMD = −1.42 [95% CI, −1.72, −1.11], P < 0.00001), and length of hospital stay (SMD = −3.73 [95% CI, −4.41, −3.06], P = 0.00001). No significant differences were found regarding transfusion requirement, operative duration, deep vein thrombosis, and pulmonary embolism between the 2 groups.
Conclusions The present meta-analysis indicates that the use of TXA in spinal surgery decreases blood loss and duration of hospital stay while not increasing the risk of side effects such as deep vein thrombosis and pulmonary embolism.
Clinical Relevance The study aims to provide clinicians who operate on spine trauma with information on the use of tranexamic acid to decrease blood loss and related complications.
Level of Evidence 1.
Introduction
Multilevel spine surgery is known to involve blood loss, although the exact definition of significant loss is unclear.1 Bleeding in spine trauma surgery can occur from the dissected paraspinal muscles, fractures, bone surfaces that have been stripped of periosteum, as well as epidural bleeding following laminectomy.2 The consequences of this blood loss remain uncertain, although the complications of consequent blood and blood product transfusions are well known. These can include transfusion-related acute lung injury, coagulopathy, immune system depression, and even increased infection rates.1,2
Multiple strategies to reduce blood product–related complications in spine surgery have been used, including autologous blood predonation, preoperative erythropoietin use, antifibrinolytics, and intraoperative cell saver technology.1
Antifibrinolytics have been used in spine surgery to minimize blood loss.3 Aprotinin and epsilon aminocaproic acid were noted to be less effective compared with tranexamic acid (TXA) in terms of total blood loss (TBL) and transfusion rates.4 TXA is a synthetic antifibrinolytic and competitively blocks the lysine-binding sites of plasmin, tissue plasminogen activator, and plasminogen. This retards fibrinolysis and hence clot degradation, thereby decreasing blood loss.5 It has been used to reduce the need for blood transfusions for more than a decade, and its safety and efficacy in trauma patients have been established.6–8
In patients with normal renal function, the half-life of TXA is 80 minutes, with recommendations of a broad range of doses in published literature.5,9 It is predominantly excreted unchanged in the urine, and dose modification should be considered in renal impairment.10,11 TXA in spine surgery is administered via topical, oral, and intravenous routes, although optimum doses are still uncertain.5,12 Intravenous tranexamic acid (iTXA) is the most commonly used route for administration with a recommended dose of 10 to 20 mg/kg bolus followed by a 1 mg/kg/h infusion, although multiple other regimens are also used in clinical practice.5,9,11 The dose of topical tranexamic acid (tTXA) is more variable and likely represents the heterogeneous surgical wounds across orthopedic and neurosurgical specialties, ranging between 1 and 3 g dissolved in saline. This is typically placed in the wound before wound closure.9,13
The most feared complication of iTXA is an increased risk of thrombotic events. Although there is a general apprehension that TXA may increase thrombogenicity, the literature does not reveal a significant increase in thrombotic events, such as deep vein thrombosis (DVT) and pulmonary embolism (PE), with most events being anecdotal and sparse.3,5,7,9,11,14 Seizures are another complication that is thought to arise from the blood-brain barrier penetration of TXA and may be due to glycine and GABA receptor inhibition, as well as possible cortical ischemia.10,15 Seizures are mainly reported to occur with high doses and have an incidence of 2.7%.9,10,16 The other complications of TXA include gastrointestinal manifestations, dermatitis, and visual disturbances.10
tTXA is theoretically poised to avoid these complications due to a lesser systemic penetration.17 However, a very real possibility in spine trauma patients is the inadvertent entry of TXA into the intrathecal space during topical application. A recent review of 21 cases of accidental intrathecal administration of TXA revealed seizure activity in all patients along with cardiac events leading to a mortality of almost 50%.15 Cardiac events such as arrhythmia may be secondary to massive sympathetic discharge during seizure activity.15 Whether TXA penetrates through intact dura mater is an interesting question. A study on TXA-soaked gelatin sponges placed epidurally postlumbar laminectomy did not show any increase in TXA-related complications, perhaps indicating poor penetration through intact dura, although further studies are needed to establish this beyond doubt.18
The use of TXA is established in spine deformity surgery and spine malignancies.9 Studies have shown its cost-effectiveness, making it a useful tool in the spine surgeon’s armamentarium.19,20 However, there is a paucity of literature involving the use of TXA in the operative management of spine fractures. This systematic review with meta-analysis was designed to understand the efficacy and safety of both intravenous as well as tTXA in the reduction of blood loss during surgery for traumatic fractures of the thoracolumbar spine.
Methods
Search Strategy
A systematic search for published studies was performed on MEDLINE, the Cochrane Library, PubMed, and Google Scholar up to 15 December, 2020. Currently registered trials were searched on the following platforms: ClinicalTrials.gov (US National Institutes of Health), International Standard Randomized Controlled Trial Number, Chinese Clinical Trial Register, and EU Clinical Trials Register. MEDLINE, PubMed, and the Cochrane Library were searched using the MeSH terms “tranexamic acid” and “spinal fractures.” The search terms “tranexamic acid” (including TXA, tTXA, iTXA, topical TXA, and intravenous TXA), “thoracolumbar fractures,” and “spine fractures” or “spinal fractures” were also used in various combinations along with their synonyms and the Boolean operators AND and OR. Study selection was restricted to studies on humans and those published in the English language. Reference lists were also examined manually to identify any initially omitted articles.
Inclusion and Exclusion Criteria
Inclusion criteria were used to identify studies that (1) focused on adult patients undergoing surgery of any type for thoracolumbar spine trauma, (2) assessed TXA as an independent variable in the achievement of hemostasis of spine trauma surgeries, and (3) provided outcome measures to assess perioperative blood loss or blood transfusions. In studies on pediatric patients, only nontrauma diagnoses as well as case reports and case series with less than 10 participants were excluded. The study was designed based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Data Extraction
Three independent reviewers manually performed data extraction (S.T., K.P., and L.R.) and analysis of published studies as well as current registered clinical trials. Any disagreement was solved by discussion or decision by the senior author (S.R.). The following data were extracted:
Demographic data: first author, country, published year, sample size, mean age, gender, and study design
Surgical/intervention data: surgical approach, dose and route of TXA administration
Surgical outcomes including TBL, postoperative blood loss (PBL), hidden blood loss (HBL), intraoperative blood loss (IBL), operation duration, transfusion rates, and postoperative complications. Other relevant data were also extracted from individual literature when necessary.
From the protocols of registered clinical trials, data were extracted according to the PICOS framework as well as inclusion and exclusion criteria.
Quality Assessment
Quality assessment was performed by 2 authors (S.T. and K.P.) using the RoB-2 tool for randomized studies as per the Cochrane Handbook for Systematic Review of Interventions and the methodological index for nonrandomized studies tool for nonrandomized trials.21,22 Publication bias was assessed using a funnel plot.
Data Analysis and Statistical Methods
Data analysis was performed using RevMan (Review Manager, version 5.4; The Cochrane Collaboration, 2020). Statistical heterogeneity was assessed using the I 2 statistic as well as the standard χ 2 statistic. I 2 > 50%, P < 0.1 was considered to imply significant heterogeneity, and the random effects model was used for analysis.23 A sensitivity analysis was conducted where necessary. Dichotomous outcomes were expressed the risk difference (RD) with 95% CIs while, for continuous outcomes, the weighted mean differences or mean differences (MD) and 95% CIs were calculated. Statistical significance was defined as P <0.05.
Results
Search Results
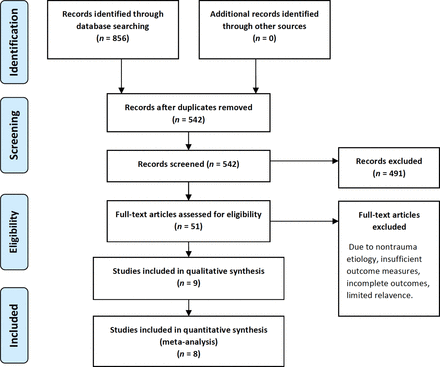
A total of 856 literature sources were reviewed. Of those articles, 314 were rejected due to duplication. After scanning titles, abstracts, and full texts as necessary, an additional 533 articles were rejected based on relevance to the topic as well as inclusion/exclusion criteria. Nine articles were included for the systematic review, and all but a single article were included for the meta-analysis due to lack of clarity in reported outcomes.24 Of these, 7 were prospective randomized trials, 1 was a retrospective cohort study, and 1 prospective observational study.24–32 Combined, the total participant strength was 828 patients. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram is shown in Figure 1. The demographic characteristics and details are presented in Table 1. Of the 155 registered clinical trials matching our search criteria, only 2 RCTs were identified and are represented in Table 1.33,34
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Studies included in the qualitative and quantitative synthesis.
Risk of Bias Assessment
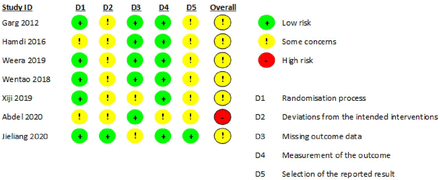
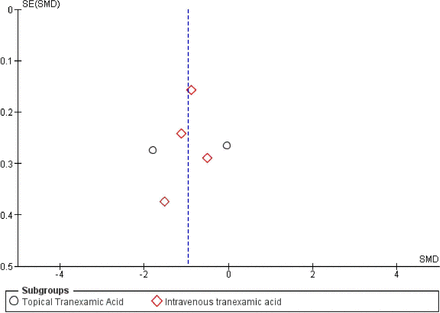
Two of the studies did not provide clear inclusion and exclusion criteria. Risk of bias analysis was performed for the randomized as well as nonrandomized studies and is displayed in Figure 2. Of the randomized studies, randomization was performed using random number generation in 2 studies, block randomization in 2 studies, and not mentioned in the others. Sealed envelope allocation concealment was reported in 3 studies, and 1 study reported only single blinding. Only 1 study reported assessor blinding. All studies reported complete outcome data. Methodological index for nonrandomized studies scores for the nonrandomized studies were 14 and 15 (Table 2).29,30 Publication bias for IBL was assessed using a funnel plot and is displayed in Figure 3.
Risk of bias table generated using the RoB-2 tool.
Funnel plot of intraoperative blood loss demonstrating publication bias. SE, standard error; SMD, standard mean difference.
Nonrandomized studies evaluated for bias using the MINORS criteria.
Study Characteristics
Completed Studies
The demographics, intervention, and study designs of the included studies are mentioned in Table 1. The surgeries performed included open pedicle screw fixation (PSF) in 5 studies, paraspinal Wiltse approach and PSF in 1 study, open PSF with interbody fusion in 1 study, percutaneous PSF in 1 study, and 1 study did not mention the type of surgery.
The study published by Garg et al was published as an abstract employing a prospective randomized trial on 52 participants and looked at the effect of a bolus dose of iTXA on blood loss.24 However, as their blood loss statistics lacked SDs and transfusion statistics were not clear, the study was not included in the meta-analysis. Hamdi et al also published an abstract detailing their prospective randomized study on the effect of TXA on patients undergoing surgeries for spine trauma.25
The study by Wang et al29 involved a comparison of different doses of iTXA to placebo. Therefore, the 2 different doses were used as 2 different studies: Feng (A) comparing intra- and postoperative iTXA to placebo and Feng (B) comparing intraoperative iTXA alone to placebo. They noted that iTXA decreases blood loss, with multiple doses further reducing the blood loss.29
Wang et al27 published a study comparing intravenous, topical, and combined iTXA and tTXA and their effects on blood loss. Although the study did not compare values to placebo, statistics were extracted and compared with either the topical or intravenous group as a control for the operative duration and HBL. They eventually concluded that while iTXA effectively decreases blood loss, tTXA does not.27
Registered Trials
The details of the 2 selected trials are represented in Table 1. Lehman et al have described their study looking into the effect of tTXA on blood loss and focus on important secondary outcome measures such as wound infection rates, cost analysis, and impact on quality of life.34 Sheng et al detailed in their protocol a randomized single-blinded study comparing the effect of iTXA and tTXA to placebo in patients undergoing open PSF surgeries.33
Outcomes for Meta-Analysis
Total Blood Loss
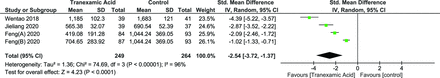
TBL was reported in 3 studies with a total of 4 groups.28,29,31 A random effects model was used due to significant heterogeneity (I 2 = 96%, P < 0.00001). A significant effect was noted in terms of TBL (standard mean difference [SMD] = −2.54 [95% CI, −3.72, −1.37], P = 0.0001) (Figure 1). No significant difference was noted on performing leave-one-out sensitivity analysis (Figure 4).
Forest plot showing the effect of tranexamic acid on total blood loss.
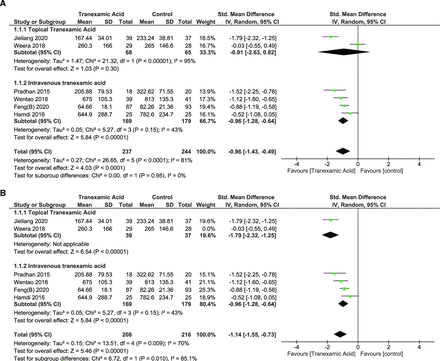
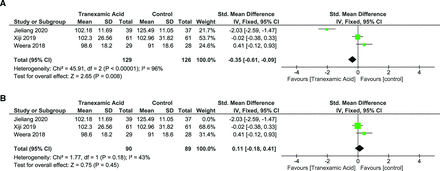
Intraoperative Blood Loss
IBL was reported in 6 studies with a total of 481 patients.25,26,28–31 Two studies used tTXA, and 4 studies used iTXA. As heterogeneity was significant (I 2 = 81%, P < 0.0001), random effects model was used. Overall analysis showed a significant positive impact of TXA over placebo (SMD = −0.96 [95% CI, −1.28, −0.64], P < 0.00001) (Figure 5A). However, these results must be interpreted with caution as one of the studies used TXA at the end of the intraoperative period and may not have substantially affected the IBL; thus leave-one-out analysis was performed.26 Heterogeneity was reduced to I 2 = 70% (P = 0.009) when the study by Weera et al was left out (Figure 5B). Subgroup analysis revealed significant heterogeneity was contributed predominantly by the tTXA group (I 2 = 95%, P < 0.00001). There was no significant heterogeneity in the iTXA group (I 2 = 43%, P = 0.15), whereas the tTXA group showed significant heterogeneity (I 2 = 95%, P = 0.00001).
(A) Forest plot showing the effect of tranexamic acid (TXA) on intraoperative blood loss. (B) Forest plot showing the effect of TXA on intraoperative blood loss after leave-one-out analysis and exclusion of Weera 2018.
Postoperative Blood Loss
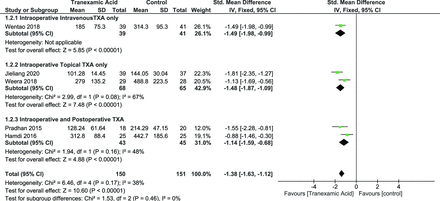
PBL was reported by 6 studies with a total of 331 patients25.26,28,30–32 Random effects model was used (I 2 = 34%, P = 0.18). The overall effect of TXA on PBL was significant (SMD = −1.42 [95% CI, −1.72, −1.11], P < 0.00001) (Figure 6).
Forest plot showing effect of tranexamic acid on postoperative blood loss.
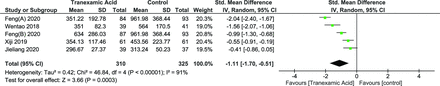
Hidden Blood Loss
HBL was reported by 5 groups in 4 studies.27–29,31 The random effects model was used due to significant heterogeneity (I 2 = 91%, P < 0.00001), which showed a significant positive effect (SMD = −1.11 [95% CI, -1.70,–0.51], P = 0.0003) (Figure 7).
Forest plot showing effect of tranexamic acid on hidden blood loss.
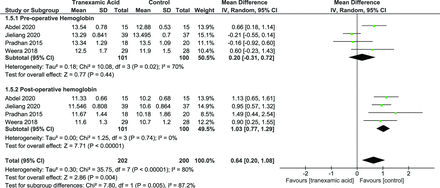
Hemoglobin Levels
Hemoglobin levels were reported by 4 studies.26,30–32 The random effects model was used, which showed a significant difference in postoperative hemoglobin (mean difference [MD] = 1.03 [95% CI, 0.77, 1.29], P = 0.00001) (Figure 8).
Forest plot showing effect of tranexamic acid on perioperative hemoglobin levels.
Operative Duration
Operative Duration With Use of tTXA
Operative duration was reported by 3 studies.26,27,31 Random effects model was used due to significant heterogeneity (I 2 = 96%, P < 0.00001). Pooled analysis showed a nonsignificant effect on operative duration overall (SMD = −0.54 [95% CI, −1.85, 0.77], P = 0.42). However, leave-one-out sensitivity revealed that excluding Jieliang 2020 from the analysis reduced the heterogeneity (I 2 = 43%, P = 0.18), although the effect remained nonsignificant (SMD = 0.15 [95% CI, −0.26, 0.56], P = 0.48) (Figure 9A and B).
(A) Forest plot showing effect of topical tranexamic acid (tTXA) on operative duration. (B) Forest plot showing effect of tTXA on operative duration after leave-one-out analysis and exclusion Jieliang 2020.
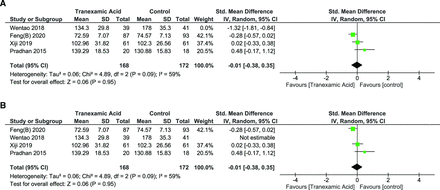
Operative Duration with Use of iTXA
Operative duration was reported in 4 studies.27–30 Heterogeneity was noted to be significant, hence random effects model was used (I 2 = 88%, P < 0.001). There was no significant change in operative duration (SMD = −0.29 [95% CI, −0.90, 0.32], P = 0.35). Leave-one-out sensitivity analysis revealed that excluding Wentao 2018 reduced the heterogeneity to I 2 = 59%, P = 0.09 with the overall effect still being nonsignificant (SMD = −0.01 [95% CI, −0.38, 0.35], P = 0.95) (Figure 10A and B).
(A) Forest plot showing effect of intravenous tranexamic acid (iTXA) on operative duration. (B) Forest plot showing effect of iTXA on operative duration after leave-one-out analysis and exclusion of Wentao 2018.
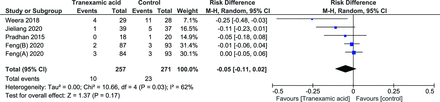
Transfusion Rate
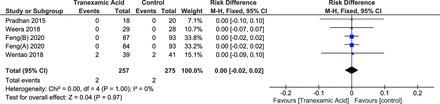
Although 1 study reported transfusion rates, 2 different sets of values were reported and due to a lack of clarity, its results were not included in the meta-analysis.24 A total of 5 groups were included from 4 studies with a total of 528 patients.26,29–31 The random effects model was used as there was significant heterogeneity (I 2 = 62%, P = 0.03). There was no significant difference in the number of patients requiring transfusion (risk difference [RD] = −0.05 [95% CI, −0.11, 0.02], P = 0.17) (Figure 11).
Forest plot showing effect of tranexamic acid on blood transfusions.
Length of Hospital Stay
Only 2 studies reported hospital stay, and both studies used tTXA.26,31 The random effects model was used (I 2 = 0%, P = 0.66), revealing a significant difference in length of hospital stay (MD = −3.73 [95% CI, −4.41, −3.06] P = 0.00001) (Figure 12).
Forest plot showing effect of tranexamic acid on length of hospital stay.
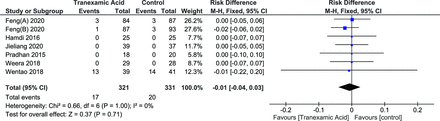
Incidence of DVT
Incidence of DVT was reported in 6 studies.25,26,28–31 Random effects model was used (I 2 = 0%, P = 1.00). The effect of TXA on the incidence was not noted to be significant (RD = −0.01 [95% CI, −0.03, 0.02], P = 0.60) (Figure 13).
Forest plot showing effect of tranexamic acid on incidence of deep vein thrombosis.
Incidence of PE
The incidence of PE was reported in 4 studies.26,28–30 Random effects model was used (I 2 = 0%, P = 1.00). The effect of TXA on the incidence was not noted to be significant (RD = −0.00 [95% CI, −0.01, 0.01], P = 0.99) (Figure 14).
Forest plot showing effect of tranexamic acid on incidence of pulmonary embolism.
Discussion
Overall Assessment
To our knowledge, this is the first meta-analysis looking into the efficacy and safety of the use of TXA in traumatic spine injuries, specifically thoracolumbar fractures. The CRASH trials have established that TXA can be used in the setting of trauma without a significant increase in prothrombotic events.7,8 TXA may have a positive effect on decreasing blood loss, although its effects on rates of blood transfusion are uncertain. Its use is not associated with an increased risk of specific adverse effects.
Dosing of iTXA and tTXA is a controversial issue, with variable recommendations in different studies.5 Although the commonly used intravenous dosage is 10 mg/kg bolus followed by 1 mg/kg/h during the study, multiple studies only use bolus doses while tTXA dosage suffers from an even higher variability.9 Only 2 studies included in our meta-analysis used a continuous infusion of TXA, while others used bolus doses. Wang et al compared 2 different dosing regimens and concluded that higher/multiple bolus dosing may lead to decreased overall blood loss, although transfusion rates did not appear to be significantly altered.29 Further, well-designed studies on the effect of timing and different doses of TXA are required.
The variability in the surgical method used in the individual studies itself leads to some bias in this analysis as they have different rates of blood loss. Xiji et al have tried to understand the effect of TXA on percutaneous PSF and concluded that iTXA can reduce IBL and TBL even though none of their patients required a transfusion.27 However, the importance of blood loss in percutaneous PSF is not clear, and more studies are needed to corroborate this finding and its clinical significance.
Blood Loss
In our review, the pooled analysis revealed that TXA makes a significant difference in blood loss in terms of TBL, IBL, PBL, and HBL. This is in keeping with conclusions drawn by other studies that have analyzed the effect of TXA in spine surgeries in general.9,13,14 Although this is reassuring, it must also be taken into consideration that there were significant heterogeneities present, which may affect the results.
The analysis of the effect of TXA on IBL bears some inspection. Although iTXA uniformly reduced IBL, tTXA only reduced IBL in 1 of the 2 studies, where it was used at an earlier point in the surgery than normally used, that is, just before wound closure.9,31 More studies are desirable to look into the optimum timing of administration of tTXA. Furthermore, blood loss is known to vary based on the surgical approach being used, which may have also contributed to the heterogeneity seen in this meta-analysis.35,36 The statistics for TBL and HBL must be taken with caution as these are both calculated estimations, which may be prone to errors. There were also some variations in the formula used in these calculations in each of the studies.
However, there was no significant change in the rates of transfusion. Nellipovitz et al stressed that blood loss is only useful as a surrogate outcome for transfusion requirements and that estimation of blood loss can be imprecise.5 The lack of effect on blood transfusion rates in our meta-analysis is interesting but in accordance with the variable results in other studies.4,37–40 Of the 5 studies that reported blood transfusions, only 3 reported specific criteria for transfusions, the variability of which may have contributed to the heterogeneity of results. There are other factors that may influence blood loss in these trauma patients, including polytrauma, disseminated intravascular coagulation, and prior use of antiplatelet or anticoagulant medication. In this analysis, only a few of the included studies have mentioned the use of anticoagulants/antiplatelet medications as an exclusion criterion. This is likely to add variability to the results. Although this device is used extensively, none of the studies has mentioned the use of bipolar sealant devices/Aquamantys for decreasing blood loss during surgery.
Operative Duration
Operative duration was reported by a total of 7 studies, 3 using tTXA and 4 using iTXA. Although there was no significant overall difference in operative duration with the use of TXA, 2 outlier studies merit attention. Jieliang et al used tTXA early on in their surgery, while most other studies use instillation toward the end of the procedure.31,41 Early use of tTXA may be an area for further research to substantiate these results. Wentao et al also showed a significant decrease in operative duration and used a continuous infusion of iTXA instead of bolus doses.28
Hospital Stay
A significant positive effect on length of hospital stay is noted, though this was only reported by studies using tTXA. Further studies using both iTXA and tTXA will be required to assess this outcome.
Adverse Events
The analysis also revealed that there was no significant positive effect of TXA on the incidence of DVT and PE. The absence of increased incidence of thrombotic events is in keeping with the findings of multiple studies.3,7,42 Other adverse effects such as seizures, stroke, and myocardial infarction were not reported even in studies using tTXA. Intact dura may prove to be a barrier to excessive neuraxial penetration of TXA, an essential prerequisite to stroke and/or seizures. In this review, studies did not include patients suspected to have a cerebrospinal fluid leak. Neurological deficits may be used as a marker for possible dural injury, and this has been used as an exclusion criterion.43,44 Whether tTXA is indicated in patients with neurological deficits is at present uncertain. Of the studies included in this review, only one specifically included patients with neurological deficits, while 3 studies excluded patients with deficits.26,27,29,31 The rest did not specify this as a criterion. At present, patients noted to have intraoperative dural tears or those confirmed on preoperative imaging may not be ideal candidates for tTXA.
Limitations
The limitations of this study include the inclusion of both randomized and nonrandomized trials for analysis, with studies having a higher risk of bias on quality analysis. The sample size is also relatively lower, with only small studies with low sample sizes included in the analysis. The included studies have used different protocols with varying surgical interventions and drug dosing regimens. Transfusion criteria were not noted to be uniform where mentioned. The inclusion criteria in this analysis also did not include the pediatric age group. Publication bias was assessed using a funnel plot, which revealed an asymmetric scatter. This may be due to several reasons, including significant statistical heterogeneity and insufficient trials in the plot leading to decreased sensitivity. Language restrictions may have also contributed to bias.
TXA may be used in the perioperative period in spine trauma to decrease blood loss without any serious side effects. Further research is required to understand the optimum dosing and timing of both intravenous and tTXA, as well as if it has any significant effect on the duration of hospital stay and costs.
Conclusion
TXA, both intravenous and topical, has become routine adjuncts in spine surgery. However, there is less experience with TXA in spine trauma surgery. This meta-analysis has reaffirmed the use of TXA in reducing perioperative blood loss and operative duration with no added risk of adverse events, although a demonstrable effect on blood transfusion rate is uncertain. Further studies in the dose and timing of TXA, as well as patient selection, are necessary.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest or financial disclosures with respect to the research, authorship, and/or publication of this article.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2022 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.