Abstract
Background While studies have identified urinary tract infection (UTI) as a complication after spine fusion, UTI is understudied in the context of fusion for spinal deformity. This study sought to determine both UTI incidence after multilevel posterior fusion for spinal deformity and whether pooled risk factors (RFs) increased UTI risk.
Methods Patients who had posterior fusion for spinal deformities between 2010 to 2019 were queried from the PearlDiver database, separated by the number of levels operated on (<7, 7–12, and >12), matched for age/gender, and analyzed for UTI incidence within 1 week and 1, 2, and 3 months. Any patient with a note of diabetes, obesity, rheumatoid arthritis, or coronary artery disease within 1-year prior to surgery and who contracted UTI within 1 month after fusion was included in the RF group for each level span. Patients of each level span with any RF were compared with those without any RFs. χ 2 tests were used for statistical analyses.
Results A total of 20,893 patients underwent posterior fusion for spinal deformities from 2010 to 2019. After matching, each level set had 2239 patients. At 1, 2, and 3 months, the >12 levels subgroup showed statistically higher UTI incidence than the 7 to 12 and <7 levels subgroups. At 3 months, UTI was similar between the <7 and 7 to 12 subgroups, with 3.8% and 3.9%, respectively (P = 0.41), and UTI was statistically higher in the >12 subgroup at 4.6% (<7 vs 7–12: P = 0.005; <7 vs >12: P < 0.001). For each level group, the RF groups had significantly higher UTI rates at 1, 2, and 3 months. ORs were significantly greater than 1 for RF groups across all level subgroups (<7 OR = 2.8, P < 0.001; 7–12 OR = 2.1, P < 0.001; >12 OR = 2.3, P < 0.001).
Conclusions Diabetes, obesity, rheumatoid arthritis, and coronary artery disease were associated with a higher risk of UTI after posterior fusion for spinal deformity for all level sets. patients who underwent procedures for more than 12 levels had the highest rate of UTI. This is the first study to analyze and compare UTI incidence following fusion for spinal deformity.
Level of Evidence 3.
Introduction
Scoliosis and kyphosis, an increase in lateral and forward curvature respectively, are spinal deformities that commonly affect the thoracic region.1 Scoliosis, the most common spinal deformity, usually affects a younger population, with 3% of 10 to 16 years old—an estimated 6 to 9 million people—affected in the United States.2–4 Kyphosis incidence, on the other hand, commonly increases with age, with 20% to 40% of adults aged 60 years or older affected and women having a greater rate of increase predominantly during menopause.1,5 Kyphosis and scoliosis both vary in the number of levels affected depending on disease severity.
While initial treatment of spinal deformity is conservative, arthrodesis procedures are indicated dependent on age, extent of curvature, and progression rate. Aims of surgery include stopping curvature progressing into adulthood, cosmetic improvement, and addressing long-term cardiorespiratory complications and/or back pain.4,6–9 The choice of fusion technique—anterior, posterior, or a combined approach—depends on many factors, including spinal deformity type, age, extent of curvature, and surgeon preference.4,7,10,11 Various studies have addressed risk factors (RFs) and complications affecting surgery outcome, focusing on either a certain subset of spinal deformity3,12–16 or a specific postoperative complication.15,17–19 A common postoperative complication is the presence of urinary tract infections (UTIs).12,13,15,16,19–21
UTIs are the most common type of nosocomial infections contracted after surgery, accounting for 23% of infections in the intensive care unit and 12.9% of health care–associated infections.22,23 UTI has been shown to significantly affect sepsis, morbidity, mortality, and cost of care.24 Indwelling catheters and surgeries with long procedure times have been proposed as RFs contributing to UTI development in previous studies.
While postoperative UTI is uncharacterized and understudied for fusion procedures specifically for spinal deformity, some studies have focused on anterior and posterior lumbar fusion, which have identified RFs significantly associated with UTI, such as older age, the female gender, alcohol use, and diabetes.25,26 Utilizing the PearlDiver Database, the aim of the current study was to identify RFs and the incidence of postoperative UTI in patients undergoing spinal fusion for spinal deformities.
Materials and Methods
Patient data from 2010 to 2019 were accessed from the PearlDiver Database (www.pearldiverinc.com; PearlDiver Inc, Fort Wayne, Indiana). PearlDiver is a national insurance database consisting of Medicare and multiple private insurance agencies. For the purpose of our study, the MSpine subset was queried using ICD-9 (International Classification of Diseases 9), ICD-10 (International Classification of Diseases 10), and/or Current Procedural Terminology (CPT) codes from quarter 1 in 2010 to quarter 3 of 2019 (Supplemental Table 1).
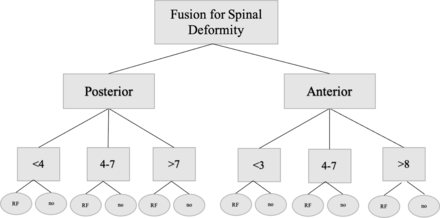
Patients were first separated based on CPT code into the anterior or posterior subcategories. Each subcategory was then split into 3 groups based on the number of levels fused. The anterior group was split into 2 to 3, 4 to 7, and >7 levels fused, and the posterior group was split into <7, 7 to 12, and >12 levels fused (Figure). Queries were structured to ensure that the 3 groups were mutually exclusive, and the pathology of neuromuscular scoliosis was excluded. Patients in each level group that contracted a UTI within 1 week, 1 month, 2 months, and 3 months were then identified using ICD-9-D codes and ICD-10-D codes. Level subgroups were matched to account for differences in the demographic characteristics of age and gender.
Query algorithm looking at subgroups of anterior and posterior fusion and then further dividing based on a number of levels fused during surgery. Abbreviations: RF: patients with set of risk factors; no = patients without set of risk factors.
Patients who contracted a UTI within 1 month postoperatively were then analyzed for the presence of various RFs existent within 1 year prior to surgery. The RFs of diabetes, obesity, rheumatoid arthritis, and coronary artery disease were identified using ICD-9-D and ICD-10-D codes (Supplemental Table 1).
An R package within PearlDiver was used to perform statistical analysis. χ 2 analysis was used to identify significant differences in UTI incidence among the 3-level subgroups and to perform further subanalysis on the effect of the chosen RFs on each cohort. A P value of 0.017 according to the Bonferroni correction was used to prevent Type 1 error.
Results
A total of 11,827 patients underwent arthrodesis procedures for spinal deformities from 2010 to 2019, with 1442 anterior and 10,385 posterior arthrodesis procedures. Matching anterior procedures gave inappropriately low sample sizes among the 3 fusion level groups. Thus, we excluded anterior procedures from our analysis and instead focused on matching and performing the initial pairwise statistics on the posterior group.
After matching for age and gender, there were 1787 patients in each of the posterior level groups. The highest incidence age range was 15 to 19 years, and the gender split was 73.4% women.
After 1 week, there was an incidence of 1.2%, 1.1%, and 0.7% for <7, 7–12, and >12 levels, respectively. No significant difference was observed in the 3 fusion level subgroups after both 1 week (<7 vs 7–12: P = 0.876; <7 vs >12: P = 0.1209; 7–12 vs >12: P = 0.21) and 1 month (<7 vs 7–12: 0.62; <7 vs >12: P = 0.3; 7–12 vs >12: P = 0.09). The >12 levels subgroup showed significantly higher UTI incidence when compared with 7 to 12 levels at 2 months at 2.2% and 3.6%, respectively (P = 0.04), and at 3 months at 2.7% and 3.9%, respectively (P = 0.04; Table 1). At the final time point of 3 months, UTI rate was similar between the <7 and 7 to 12 level groups, with rates of 3.6% and 2.9%, respectively (P = 0.15), and statistically higher in the >12 levels group, with a rate of 3.9% (7–12 vs >12: P < 0.04). The highest UTI incidence increase occurred within the first 7 days for the <7 and 7–12 levels groups, and from 1 week to 1 month for the >12 levels group.
UTI incidence in patients undergoing posterior arthrodesis with either <7, 7–12, or >12 levels fused.
As expected, the RF group had significantly higher UTI rates at 1, 2, and 3 months for all fusion level groups (Table 2). The largest risk difference was seen at 1 month for <7 levels, 3 months for 7–12 levels, and 2 months for >12 levels. Patients who underwent <7 levels fusion were 2.73 times more likely to contract a UTI if they had a RF (P = 0.01); patients who underwent 7 to 12 levels fusion were 2 times more likely (P = 0.01); and patients who underwent more than 12 levels fusion were 2.8 times more likely (P < 0.001). The largest difference in UTI incidence between patients with and without RFs was at 3 months in all level groups: <7: 5.5% vs 2.4% (OR = 2.4; P = 0.004); 7–12: 3.8% vs 2.0% (OR = 2.0; P = 0.01); >12: 6.4% vs 2.8% (OR = 2.4; P = 0.002). Significance and OR comparing the time point of 7 days are not included because patients within the RF and non-RF groups totaled future than 11 patients within all 3 fusion level subgroups.
UTI rate of patients who underwent posterior spine fusion and who either had or did not have RFs.
Discussion
The current study showed that patients with diabetes, obesity, rheumatoid arthritis, and coronary artery disease had a significantly higher risk of contracting UTI than patients without, regardless of the type of procedure or number of levels fused. In addition, within the posterior group, a greater number of levels fused had significantly more UTI cases. Spinal deformities, such as scoliosis and kyphosis, are disorders that can appear in all age ranges, most commonly arising during adolescence or progressively worsening due to age.2–4 Fusion may be indicated when curvatures exceed 45° or more than 70° with pain in the cases of scoliosis and kyphosis, respectively.4,6,7 Multiple previous studies have demonstrated UTI as a common postoperative complication affecting surgery success.12,13,15,16
UTI incidences 1 month after either anterior or posterior procedures were higher than previously reported. Bekelis et al reported a 1.37% incidence in general spinal surgery patients (including anterior surgery, corpectomy, and fusion).27 Similarly, 2 separate studies reported UTI contraction in 1.41% of patients who underwent posterior lumbar interbody fusion and 1.77% of patients who underwent anterior lumbar interbody fusion (ALIF).25,26 In contrast, a study analyzing elective spinal surgery reported an even lower UTI incidence rate of 0.88% in ALIF patients.23 The higher incidences of UTI demonstrated in our study are consistent across the different spinal deformity levels. This may be due to the higher number of female patients in the cohort, as young women have been shown to have high incidences of UTI contraction.
Patients who had spinal fusion with >12 levels showed a statically greater percentage of UTI cases than patients who had spinal fusion for 7 to 12 levels. This distinction may be due to the fact that procedures requiring more levels fused are more involved, longer, and require longer recovery length of stay in the hospital, increasing the opportunity of bacterial exposure.16,25,28,29 Procedure length is an identified RF for UTI, characterized in both spinal and nonspinal surgery-related settings.28,30 A comprehensive retrospective study of 1,452,369 patients who received general anesthesia for unspecified surgery was identified using the American College of Surgeons National Surgical Quality Improvement Program database. The 2 highest quintiles for procedure length showed an increased risk with OR of 1.156 and 1.758, as compared with the third quintile for reference.30 In addition, in the context of spinal surgery, analyses of UTI incidence in ALIF and posterior lumbar interbody fusion patients also recognized longer operative time as an associated RF.25,26
In our dataset, the highest number of UTI cases appear within the first 7 days postoperatively for all level groups except for <12 levels fused during the posterior procedure, which has the highest number from 1 week to 1 month. While there are cases that appear after 1 month, there is a sharp decline of cases after this point, at both the 2- and 3-month time points. This timing of UTI onset is consistent with values previously reported for spinal surgeries. Median postoperative day of UTI occurrence in previous studies range from 2 weeks to 1 month.23,26
The current analysis included prospective matching for age and gender in the posterior arthrodesis group. Female gender and sexual activity have been shown to be RFs of UTI. Both factors are well described in existing literature. Women have shorter urethras, allowing for shorter distances that bacteria must travel to infect the bladder.31,32 Displacement of pre-existing vaginal uropathogens to the bladder during sex has been proposed as an additional contributor to increased UTI risk in women.33 Multiple studies have shown a strong association between sexual intercourse frequency and UTI, with a pronounced rise in cases in women beginning sexual activity as opposed to children and adolescents.31,33–37 Our cohort of arthrodesis patients retrospectively analyzed were younger, in the postadolescent age range of 15 to 19, and were more than 2/3 women. Again, the large ratio of young women in our cohort may have contributed to the noted high rate of UTI.
As anticipated, the included RFs led to a significantly higher incidence of UTI at each of the time points in each of the 3 groups.
Diabetes mellitus, coronary artery disease, and obesity are all well-characterized RFs for UTI in both spinal and nonspinal surgeries. Patients with diabetes have been shown to be more susceptible due to their impaired immune system and the common complication of diabetic neuropathy, leading to delayed and incomplete bladder emptying.38,39 In addition, obesity and coronary artery disease have been shown to be comorbidities associated with infection as well.40–43 While the link to obesity is largely unknown, it is believed that systemic metabolic dysfunction may encourage UTI occurrence.43
The RF of the underlying disease of rheumatoid arthritis, while described in nonspinal surgeries, has not been included in UTI contraction postspinal surgery studies. Rheumatoid arthritis patients have been shown to have increased susceptibility to many serious infections due to impaired adaptive cellular immunity, neutropenia, and other interruptions to the immune system.44–46
While the present study is the first to our knowledge to characterize UTI in the context of surgery for spinal deformities, the present study has some limitations, with the main limitation being isolating the spinal location of the actual procedure. Despite the CPT codes grouping the thoracic procedures, it is unclear whether the surgery spanned the cervicothoracic or thoracolumbar spine, especially given the large number of levels operated on. Furthermore, it is important to note that while we did not separate our cohort based on each pathology due to the limitation of sample size, most of the population was in the idiopathic rather than the de novo cohort. In addition, while our initial intention was to separate individual RFs, small sample size led to the decision to pool all RFs together. Lastly, there are other comorbidities and complications that may not have been accounted for, despite being associated with UTI.
Conclusion
Diabetes, obesity, rheumatoid arthritis, and coronary artery disease were associated with an increased risk of UTI following arthrodesis for spinal deformity, regardless of the number of levels. In addition, fusion procedures with more than 12 levels had the highest rate of UTI. This is the first study to analyze and compare UTI incidence following arthrodesis on different level lengths for spinal deformity.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
Disclosures Jeffrey C. Wang reports royalties or licenses from Zimmer-Biomet, DePuy Synthes, and Seaspine; consulting for PrecisionOS (no financial payments); payment for expert testimonial from various law firms; support for attending meetings/travel and leadership role with honorarium from the AO Foundation Board; stock/stock options from Bone Biologics, PearlDiver, and electroCore; fellowship funding from AO Spine North America (paid to institution); and serving as the editor in chief of Global Spine Journal (no payment). Raymond Hah reports grants or contracts from A-TEC and SI-Bone (paid to institution); royalties/licenses and consulting fees from NuVasive; and serving in leadership role for the North American Spine Society, Lumbar Spine Research Society, and Korean American Spine Society. The remaining authors have no disclosures.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2025 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.