Abstract
Purpose Degeneration of the intervertebral disc is considered to be central in pain pathogenesis in patients suffering from chronic low back pain (LBP). In recent years, the injection of mesenchymal stromal cells (MSCs) into the disc to arrest or reverse the degenerative process has been proposed as an alternative therapy. The aim of the present study was to investigate the feasibility of using iron-labeled MSCs for intradiscal injection in patients with long-standing LBP.
Methods Ten patients (7 men, 3 women, mean age 40 years, range 26–53) with chronic LBP and confirmed disc degeneration on magnetic resonance imaging (MRI) were recruited from the waiting list for planned surgery. Injection of autologous, expanded, and iron-labeled bone marrow–derived MSCs (BM-MSCs) into 1 or 2 disc levels was undertaken. Follow-up consisted of monitoring of adverse events, regular MRI examinations, and collection of patient-reported outcome measures (PROMs) for a minimum of 2 years.
Results No complications could be detected, neither clinically nor on MRI. No statistically significant improvement was seen for PROMs on a group level up to 2 years postinjection. Three of 10 patients opted to proceed with the initially planned surgery within the first year and 2 more within 3 years postinjection.
Conclusion Results from this pilot cohort study show that injection of autologous expanded iron-labeled BM-MSCs is a safe procedure, in accordance with the existing body of evidence. The clinical result warrants further larger studies.
Level of Evidence 4 for therapeutic studies.
INTRODUCTION
Low back pain (LBP) is well recognized as a major cause of disability worldwide.1 The exact source of pain remains unclear; however, it is widely accepted that degeneration of the intervertebral disc (IVD) is central in the development of LBP.2 Current treatment modalities include several nonsurgical options, and for patients with refractory pain, surgical intervention may be considered.3 None of these treatment options guarantees pain relief; therefore novel, more biological approaches have been suggested.4 In vitro coculture studies of mesenchymal stromal cells (MSCs) and nucleus pulposus cells have shown promising results with regard to extracellular matrix production.5,6 In vivo studies in animal models have shown injection of MSCs to the IVD to be feasible and that MSCs can survive in the disc for several months.7–9
A few small cohort studies in humans using MSCs for injection into IVDs have been reported, most with limited follow-up time.10–14 No major safety considerations have been reported.15 Both bone marrow–derived MSCs (BM-MSCs) (autologous and allogeneic) and adipose tissue–derived MSCs (AT-MSCs) (autologous) have been used. A large-scale trial utilizing allogeneic BM-MSCs is registered as ongoing.16 There are several unanswered questions including whether the MSCs survive in the human IVD and how patients with long-standing pain respond to intradiscal cell injection.
The primary aim for the present study was to investigate the feasibility of intradiscal injection of autologous, iron sucrose–labeled BM-MSCs in a group of patients with more than 2 years of LBP and recruited from the waiting list for either fusion or total disc replacement (TDR) surgery. We report on the cell culture and evaluation process, possible clinical and radiological adverse events, and clinical results for a minimum of 2 years.
PATIENTS AND METHODS
Patients
Patients on the waiting list for either transforaminal interbody fusion (TLIF) or TDR due to degenerative disc disease were recruited. The decision for TLIF or TDR was made at the outpatient clinic of our hospital by a specialist spine surgeon and was irrespective of the study. The authors screened thereafter the waiting list for eligible candidates. Inclusion criteria were age 18 to 65 years, severe LBP refractory to nonsurgical treatment, no pain radiation below knee level, and degenerative disc disease at 1 or 2 lumbar spine levels. Patients included in the study should be eligible for both TLIF and TDR, thus patients with collapsed disc height >50% or evident arthrosis of the facet joints were not considered. Exclusion criteria were rheumatoid arthritis or other serious comorbidities.
The study was approved by the regional ethical committee (VGR 505–12) and the Swedish Medical Products Agency, and written informed consent obtained from all participants. At the 6 months’ time point patients could opt to proceed with the originally planned surgical procedure. At such surgery, the IVD tissue of the treated level was harvested and examined (data presented separately).17
MSCs Harvest and Cell Culture and Preparation
Percutaneous bone marrow aspiration (BMA) was performed under local anesthesia and sterile conditions with a 14.5G bone biopsy needle from the posterior iliac spine and tested for HIV, hepatitis virus A and B, and syphilis. Autologous serum was collected and used during the ex vivo expansion and implantation.
The BMA was centrifuged in liquid density gradient medium (CPT tubes with FICOLL, BD, Franklin Lakes, NJ, USA), mononuclear cells isolated and thereafter seeded in plastic culture flasks at the density of 150 to 250 × 103 cells/cm2. DMEM-LG (Dulbecco’s Modified Eagle’s Medium-Low Glucose) culture medium was used with addition of gentamycin, amphotericin-B, L-glutamine (all from Thermo-Fischer Scientific, Waltham, MA, USA), b-FGF (10 ng/mL) (R&D Systems, Minneapolis, MN, USA), and 10% autologous serum. The flasks were incubated at 37°C, 7% CO2, and 90% humidity. The cells were passaged at approximately 90% confluency. No antibiotics were used in the final passage. Fluorescence-activated cell sorting (FACS) analysis was performed on cells isolated from BMA and further on iron-labeled cells intended for implantation using a FACSAria II (BD, Franklin Lakes, NJ, USA). Cell samples were screened for CD34, CD45 (markers of hematopoietic cell lineage); and CD90, CD105, and CD166 (markers of MSCs). The presence of double positive cells for CD34/CD45 and CD105/CD166 was examined as well as the percentage of CD90+ cells within the CD105+/CD166+ population. The percentage of dead cells was assessed with the 7-AAD (7-amino-actinomycin D) method. Antibodies for CD105 and CD166 were from AbD Serotec, Kidlington, UK, otherwise from BD, Franklin Lakes, NJ, USA.
Iron labeling was performed in order to enable future tracing of the cells in explanted IVDs from patients that might choose to be operated upon as originally planned, as separately reported.17 The labeling was performed 2 to 3 days prior to scheduled implantation according to a previously published protocol using Venofer (iron sucrose) (Vifor Pharma Nordiska, Solna, Sweden) at a concentration of 1 mg/mL in the medium.18 After 16 to 20 hours of incubation, the culture medium was changed. Before injection, the cells were suspended at a concentration of 1 × 106 cells/mL in F12 medium (Thermo Ficher Scientific, Waltham, MA, USA), 20% autologous serum, without antibiotics.
Samples from the last medium change and from the cell preparation for injection were examined by bacterial cultures before release for implantation. Cell viability >90% tested with trypan blue test was further required.
Both iron-labeled and unlabeled cells were additionally cultured in a pellet mass culture system as previously described18 to ensure that the injected cells maintained their functionality ex vivo. The pellets were harvested after 7, 14, and 28 days, fixated, paraffin imbedded, sectioned (5–7 μm) and stained with Alcian blue Van Gieson. The ability of the cells to form pellets and the morphology of these were assessed by light microscopy (Nikon Eclipse E600, Nikon Japan). Due to the iron labeling of the cells scoring with Bern score as originally planned was not possible.
Injection of MSCs
The implantation of MSCs was performed with a 22G discography needle, under sterile conditions in an operating theater, after infiltration of the skin with local anesthetic and after a single dose of intravenous antibiotics (Cloxacillin, 2 g, Stragen Pharma, Geneva, Switzerland). The placement of the needle tip in the center of the nucleus was confirmed by fluoroscopy in 2 planes and a total of 1 × 106 cells in a 1 mL suspension was injected/disc level. The L4-L5 level was injected in 8 patients, the L5-S1 in 1 patient, and both these levels in 1 patient.
Follow-Up
Follow-up consisted of lumbar MRI examinations and patient-reported outcome measures (PROMs) questionnaires pre-operatively and 3, 6, 12, and 24 months after the injection. An additional MRI was obtained within a week after the intervention.
The PROMs used were the European quality of life-5 dimensions (EQ-5D 3L), the Oswestry Disability Index (ODI) and the visual analog scale (VAS), and Numerical Rating Scale (NRS) for low back and leg pain. Since the follow-up questionnaire package was adopted from the SWESPINE register and VAS was changed to NRS, during the study period some of the early questionnaires included the VAS while the later had the NRS. These 2 pain scale variants are highly correlated, not interchangeable though.19 Obtained observations in VAS were converted, by dividing by 10 and rounding up to a whole number, to NRS by the investigators.
The MRI investigations were performed with 1.5 Tesla scanners (Philips Healthcare, Best, Netherlands) and T1- and T2-weighted sequences obtained. The MRIs were reviewed as part of clinical routine by the hospital’s radiologists with focus on morphological changes over time to ensure that no radiological adverse effects occurred.
Statistical Analysis
Mean values and 95% confidence intervals were calculated for each PROM parameter/time point for the whole cohort. Friedman 2-way analysis of variance by ranks for related samples was used for comparison of PROMs between different time points. The level of statistical significance was set at 0.05. The SPSS (version 25) (IBM Corp., Armonk, N.Y., USA) statistical package was used.
RESULTS
Patient Characteristics
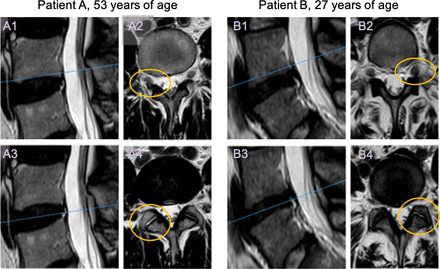
Ten patients, 7 males and 3 females with a mean age of 40 years (range 26–53) were recruited. All patients reported pain duration for >2 years. Mean body mass index was 26.1 kg/m2 (range 21–34). Five patients were working full-time (light or moderate physical work), 1 part-time, and 4 patients were on sick leave. One patient reported no use of analgesics, 5 intermittent, and 4 regular use. Two patients were smokers. All the treated IVDs were classified as grade 3 or 4 according to the Pfirrmann classification on MRI at baseline (Figure 1).
Representative images from baseline T2-weighted MRI controls from 2 patients. The L4-L5 intervertebral disc was addressed in both. To the left (A1, A3, B1, and B3) midsagittal images. The blue lines represent the level of the axial images shown to the right (A2, A4, B2, and B4). On the upper row (A2 and B2) the foramina can be evaluated, and on the lower row, the facet joints can be seen (indicated unilaterally by ellipses in orange).
Characteristics of the Injected Cells
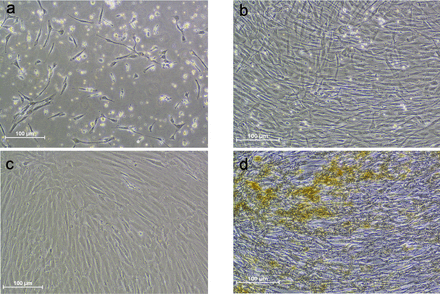
Bone marrow of mean 43.4 mL, (range 40–51 mL) was aspirated from the patients. The cell count directly after isolation demonstrated a wide range of 0.65 to 3.8 × 106 mononuclear cells/mL (mean 2.38 × 106 cells/mL). The mean expansion time was 25.9 days (range 21–44) and the cells underwent 1 to 3 passages before injection (Figure 2). The time from BMA to disc injection was determined by the availability of cells needed for the procedure.
Bone marrow–derived mesenchymal stromal cells (BM-MSCs) in culture. Cells from the same patient at different time points. (A) BM-MSCs after 6 days of culture. (B) BM-MSCs after 12 days of culture. (C) BM-MSCs prior to iron labeling. (D) Cells just prior to injection.
FACS analysis showed few cells at isolation expressing MSC surface markers, whereas at implantation this number was highly increased, and the opposite was seen for expression of hematopoietic lineage surface markers (Table 1). At implantation, 58.8% (SD 16.4) of cells were CD105+/CD166+ and of these 98.1% (SD 1.1) were CD90+. Cell viability (trypan blue) prior to implantation was 98.9% (SD 0.99) and dead cells (7-AAD method) 5.1% (SD 2.8).
Fluorescence-activated cell sorting analysis of the cell isolate after bone marrow aspiration and of the cell population for implantation.
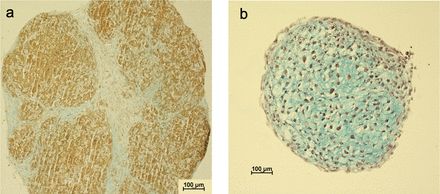
Iron-labeled cells formed somewhat more brittle pellets compared to pellets from unlabeled cells with less proteoglycan accumulation as visually observed at histology sections (Figure 3). No major differences were seen between the patients regarding the ability of the cells to form pellets.
Representative images of histological sections of bone marrow–derived mesenchymal stromal cells pellets harvested at day 28, stained with Alcian blue Van Gieson. (A) Pellet from iron-labeled cells. (B) Pellet from nonlabeled cells. The blue color represents proteoglycans and black dots signify cell nuclei. As can be observed, proteoglycan accumulation is more evident in the nonlabeled cells.
Complications and MRI Follow-Up
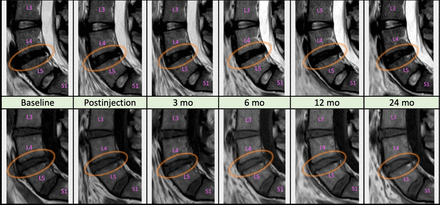
No complications were reported. For 1 patient, the first planned cell injection was canceled due to suspected cell culture contamination, and the aspiration and cell culture procedure were repeated. MRI investigations showed no signs of infection, tumorigenesis, or structural deterioration in the affected or adjacent segments at any time point for any patient (Figure 4).
Magnetic resonance imaging examinations of the same patient throughout the duration of the study (mo = months). Midsagittal sections, T2W images on top row, T1W on bottom. The 3 distal lumbar and the first sacral vertebrae are marked (L3-5 and S1). Injection of mesenchymal stromal cells was performed at the L4-L5 level (encircled). Routine evaluation revealed no evident morphological changes.
Clinical Outcome
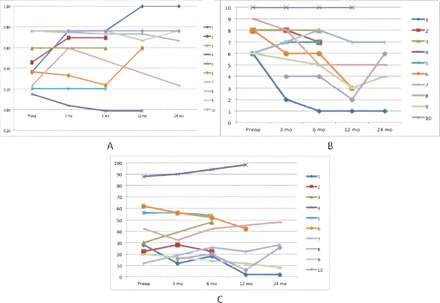
The comparison of the registered PROMs between the different time points is reported on a group level in Table 2. No statistical differences between time points were observed. Numerically most PROMs improved over time. The individual PROM scores are shown in Figure 5. One patient did not complete the PROMS prior to the injection. Two patients were not available for clinical evaluation at the 3 months’ time point.
Diagrams depicting results for (A) European quality of life-5 dimensions index. (B) Numerical Rating Scale for back pain. (C) Oswestry Disability Index for all patients over time. Patients 2, 3, and 5 opted for surgery within 1 year, patient 6 within 2 years, and patient 4 within 3 years.
Results of patient-reported outcome measures at different time points, presented as mean value and 95% confidence interval.
At the 6 months follow-up, 3 patients reported no amelioration and proceeded with the initially planned surgery within 12 months from the cell injection. Furthermore, 1 patient was scheduled for surgery within 1 year but opted to postpone it for almost 3 years after the injection of MSCs. One patient chose to proceed to surgery 2 years after the injection procedure. One patient sustained a tibial fracture 5 months postinjection and due to the development of a severe pain syndrome was unable to complete all the planned follow-ups.
No relationship between the number of cells yielded by the BMA, the time of the ex vivo expansion, the number of cell passages, and the clinical outcome was seen when comparing the group of patients that proceeded with surgery with the patients that did not.
DISCUSSION
In the present feasibility study, autologous, expanded, BM-MSCs, labeled with iron sucrose, were injected into degenerated IVDs in an attempt to ameliorate symptoms and postpone or avoid planned surgery. No complications or adverse effects were recorded.
The cell culture procedure demonstrated that a time period of about 3 to 4 weeks was needed to obtain the planned number of cells with predominantly MSC characteristics.
Seven out of 10 patients postponed planned surgery for more than a year and 5 out of 10 chose to not proceed to surgery for at least 2 years after the intervention.
The PROMs for the group showed numerically some improvement; however, no statistically significant changes were seen over time. Neither did we see any correlation between the cell yield from the aspiration, time of ex vivo expansion, number of passages or ability of the cell to form pellets, and the clinical outcome. The study was however neither designed nor powered to detect such differences. The results from this cohort support previous findings that injection of MSCs to degenerated IVDs is a safe procedure as no complications or adverse events were recorded. The detection of implanted cells from explanted disc from this patient cohort has previously been reported.17
A few similar studies introducing autologous BM-MSCs into degenerated discs have been reported.11–14,20 The patient groups differed between these studies, both regarding inclusion of patients with or without radiating pain and symptom duration. There is further a large heterogeneity regarding the source of employed cells, isolation and expansion methods, number of implanted cells per disc (between 105 and 4.5 × 107), and implantation medium (or carrier).
The differences extend to the tools used to evaluate the treatment effect and the follow-up time varies between 1 and 6 years. This makes attempts to compare results between studies difficult. In the randomized trial by Noriega et al,12 injection of allogeneic BM-MSCs was compared with a sham procedure. A subgroup of the treated patients (5/12) was reported to respond well in terms of pain relief, whereas the rest showed results comparable to those of the control group. In the study by Orozco et al,11 a pain improvement of 71% was reported. However, pain relief was not as evident in the present study. This may be attributed to patient recruitment from the surgical waiting list after failed nonoperative treatment and long symptom duration ( >2 years). The latter has recently been shown to affect other LBP treatment modalities.21 Furthermore, the iron sucrose labeling used might have affected the MSCs’ performance, since in a previous in vitro study,18 this labeling somewhat delayed the onset of glycosaminoglycan synthesis. However, for all reported disc cell injection studies, pain relief has been reported to be between 50% and 90%, so the present study is overall relatively well in line with those.
Another parameter of interest in designing this type of study is the number of cells used for injection. In the present study, the cell number was based on data obtained from preclinical and animal studies9,22 and taking into account that a mature nucleus pulposus contains approximately 4 × 106 cells/cm3.23 Too few cells could lead to insufficient therapeutic effect and injecting too many could lead to cell death due to nutrient deprivation.22 With the exception of the study by Yoshikawa et al,20 a higher number of cells have been used in other studies.
Preclinical studies further indicate that cell senescence is induced at high passage numbers.24 The cells in the present study were of passage 1 to 3. In the previously reported clinical studies, the number of passages varied between 0 and 5.10,11,20,25
The injected MSCs in the present study were suspended in expansion medium alone. The use of some kind of “carrier” has been proposed as a means to enhance survival and functionality of the cells.26 The safety and efficacy of such a combined approach has been addressed in the studies of Yoshikawa et al20 and Kumar et al.10 There are as yet no reported studies in humans comparing the effect of intradiscal injection of MSCs alone to that of an injection with a carrier.
MSCs in the present study were expanded with the use of autologous serum. In the literature, the use of serum-free media is proposed for large-scale manufacturing of cell products27 in order to avoid the use of fetal bovine serum. The use of autologous serum poses logistical problems that are manageable, though in small-scale studies as the present one.
The presented study was designed and approved as a safety and feasibility one, therefore no control group was included. The small number of patients included and the absence of a control group are of course a clear limitation of the study, as it does not permit any robust statistical analysis of the results. However, patient uniformity with regard to pain duration (longer than 2 years) and distribution (absence of radiating pain below the knee), characterization of the injected cells, and the minimum of 2 years follow-up add to the strength of this investigation and form the basis for designing randomized studies with this setup.
CONCLUSION
This study adds to the body of evidence indicating that disc injection of autologous, expanded MSCs is a feasible and safe procedure. To the best of our knowledge, this is the first study applying the concept of injecting autologous MSCs in patients who have already conceded to surgical intervention. The fact that 5/10 patients could forgo surgery indicates that this procedure deserves further evaluation as a plausible therapeutic alternative, bridging the gap between currently used nonsurgical and surgical interventions. Larger-scale studies are needed to further elucidate optimal patient selection as well as optimization of cell-related factors.
Acknowledgments
The authors would like to thank all the personnel at the accredited Cell and Tissue Laboratory at the Department of Clinical Chemistry, Sahlgrenska University Hospital, for their excellent skills and good collaboration.
Footnotes
Funding The study was financed by grants from the Swedish state under the agreement between the Swedish government and the country councils, the ALF agreement ALFGBG-772931 and ALFGBG-594211, The Swedish research council, the Ingabritt and Arne Lundberg foundation, the Dr. Felix Neubergh foundation, the Hjalmar Svensson foundation, The Gothenburg Society of Medicine, BIOMATCELL VINN Excellence Center of Biomaterials and Cell Therapy/ University of Gothenburg, the Sahlgrenska university hospital research funds.
Declaration of Conflicting Interests The author(s) report no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributors H.B., H.H., H.B.H., and A.L. designed and planned the study. N.P., H.B., A.B., and H.H. were responsible for patient recruitment, patient information, cell injections, and clinical follow-ups. A.L. was responsible for the laboratory process of cell expansion and preparations of cell implants. HH was responsible for MRI follow-ups. H.B.H., D.H., and N.P. performed and interpreted the result from the pellet cultures. N.P. and H.B. drafted the first manuscript and all authors read and approved the final manuscript.
Patient Consent for Publication All participants provided written informed consent.
Ethics Approval The study was performed after ethical approval from the Regional Human Ethical Review Board VGR 505-12 and approval from the Swedish Medical Products Agency.
Data Availability Statement All relevant data are included in the manuscript.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2021 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.