Abstract
Background Spinal tuberculosis (TB), or Pott’s disease, is the most common form of osteoarticular TB. Early diagnosis and anti-TB drug therapy are the mainstays of treatment. However, in advanced stages, surgery is essential to correct spinal deformities and avoid neurological damage. Very few cases in young children requiring surgical treatment have been described. We present 2 cases of surgically treated thoracic spinal TB in patients under 2 years of age and review the literature on this entity in young children.
Case Description Two male toddlers, aged 21 (patient 1) and 23 (patient 2) months, were admitted due to insidious systemic deterioration associated with neck stiffness and nonspecific abdominal pain in patient 1 and limping in patient 2. The findings of laboratory tests were unremarkable despite an increase in acute-phase reactants. After an extensive workup, results of a magnetic resonance imaging were consistent with spinal TB in both patients. Patient 1 underwent costotransversectomy of the left third and fourth ribs, debridement, T3-T4 corpectomy, and T2-T6 posterior fusion, to which an anterior rib autograft was added. Patient 2 had a right-sided thoracotomy performed at the apex in addition to debridement and T8 corpectomy; the resected rib was used as anterior structural autograft. Several samples of purulent, caseous material were sent for study. On follow-up, progression of proximal junctional kyphosis was evidenced in patient 1, requiring revision surgery to restore sagittal alignment; the patient was asymptomatic at 2-year follow-up. Patient 2 evolved satisfactorily without neurological sequelae and was asymptomatic at 4-year follow-up.
Conclusions Spinal TB in young children requires appropriate surgical treatment when presenting at advanced stages or having severe deformity or neurological compromise. Despite the challenges associated with young age and ongoing growth, surgery can be performed safely provided proper surgical expertise. After extensive debridement and decompression, priority should be given to restoring sagittal alignment and balance with stable and durable spinal reconstructions.
Level of Evidence 3.
INTRODUCTION
Spinal tuberculosis (TB), also known as Pott’s disease or tuberculous spondylitis, is the most common manifestation of osteoarticular TB. The disease accounts for about half of all TB cases involving the skeletal system1,2 and around 10% to 15% of extrapulmonary TB (EPTB) cases3 and it is the third most common type of EPTB.2 There are many predisposing factors and population groups with increased risk of spinal TB, such as immunocompromised people, children, the elderly, and immigrants from endemic countries.4
Affecting about a quarter of the world’s population, TB is a global health problem and a significant cause of morbidity and mortality worldwide.5 Although it is endemic in many developing countries, an increase in TB has been observed in the developed nations, due in part to immigration.3,5 In 2018, adult men were affected more frequently than women (57% vs 32%), and children accounted for 11% of cases.5 Men are also at slightly higher risk of developing spinal TB.6 EPTB may follow a severe clinical course due to hematogenous or direct disease spreading.2 Also, spinal TB in children is more aggressive than in adults and usually involves multiple levels of the spine.7
Spinal TB may have an insidious onset and is often difficult to discern in differential diagnosis, which usually causes long delays in diagnosis.2 The disease usually manifests as back pain with some associated systemic TB symptoms, such as intermittent fever, night sweats, or weight loss.3 Due to the nonspecific clinical presentation of the disease, a high index of suspicion is essential. TB diagnosis is often confused with malignancy.2,8 Concomitant pulmonary involvement may not be present, further complicating the diagnosis.2 Spinal kyphotic deformity and neurological symptoms may occur in advanced cases.
Magnetic resonance imaging (MRI) is the most sensitive imaging method for the diagnosis of spinal TB, and characteristic findings include vertebral-body destruction, anterior wedging, and paravertebral abscesses.6,9,10 Thoracic vertebrae are the most commonly involved site, followed by the lumbar spine and the cervical region.11 In most patients, correct diagnosis is established by microbiological and/or histopathological examination of the affected tissues. Samples can be obtained by percutaneous biopsy or via intraoperative means.
Early diagnosis and appropriate treatment with an adequate regimen of anti-TB drugs is the core of spinal TB treatment and can prevent the development of sequelae.2,12–15 However, in advanced stages, surgery may be necessary to correct spinal deformities and avoid potential neurological damage. Indications for surgery include kyphosis of greater than 30° to 40° or progressive kyphosis, significant vertebral-body destruction, neurological deficits, or disease progression due to spinal cord compression, poor response to anti-TB drugs, drainage of abscesses, or spinal instability.16
Most existing studies on spinal TB have reported a greater frequency among adults, with fewer cases involving children. Moreover, very few spinal TB cases requiring surgical treatment have been described in patients younger than 2 years. Herein, we present 2 cases of male infants who had advanced thoracic spinal TB. The diagnostic process, surgical treatment, and evolution are described. Additionally, the current literature and reported cases of surgically treated spinal TB in infants are reviewed.
PATIENT 1
A 21-month-old boy born in Spain and with no available medical records was brought to the hospital by his parents, who were Dominican nationals. The parents reported a 2-week history of general deterioration, decreased activity, and poor appetite. On examination, the patient was febrile, presented neck lateralization, pain upon cervical mobilization, and local tenderness, and he complained of unspecific abdominal pain. Cranial computed tomography (CT) and abdominal ultrasonography images were inconclusive. Laboratory tests showed a slightly elevated white blood cell count. He was admitted for study, and empirical antibiotic therapy was initiated. Due to the persistence of symptoms, fever, and increased acute-phase reactants, an MRI scan of the cervicothoracic spine was performed, revealing a tuberculous lesion located at T3 and T4 with T1-T5 paravertebral soft-tissue involvement and epidural abscess with medullary canal compromise and no signs of myelopathy (Figure 1).
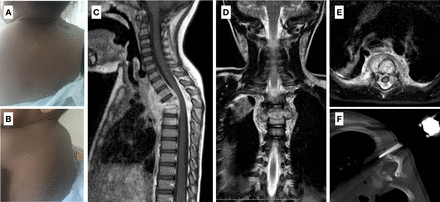
(A and B) Clinical images of patient 1, showing a marked upper thoracic kyphosis. (C–E) Sagittal, coronal, and axial magnetic resonance imaging (MRI) images of the cervicothoracic spine: bony destruction at the T3 and T4 vertebrae, causing collapse and anterior wedging (sagittal Cobb on MRI of 34°), associated substantial paravertebral soft-tissue extension (an abscess is seen between the T1 and T5 vertebral bodies), and epidural abscess, resulting in spinal canal narrowing and cord compression with no signs of myelopathy. (F) Image of the percutaneous computed tomography-guided biopsy of the involved paravertebral soft tissue.
A percutaneous CT-guided biopsy was performed, and a tuberculin skin test (TST, Mantoux reaction) and gamma interferon release assays were positive for mother and child. Therefore, the diagnosis of spinal TB was established, antitubercular medication was started, and surgical treatment was scheduled.
Under general anesthesia and neurophysiological monitoring, the patient was placed in a prone position. Using a left three-fourth Capener’s costotransverse approach, the upper thoracic spine was exposed, revealing abundant exudative, caseous material. Thorough debridement of the infected tissues was performed, and the remaining devitalized vertebral bodies were resected, resulting in a T3 and T4 corpectomy. Several samples of the infectious material were sent for microbiologic and histopathologic study. A rib autograft was used for anterior support. Subsequently, deformity correction and stabilization were achieved by a T2-T6 posterior spinal fusion with pedicle screws (Figure 2A and B).
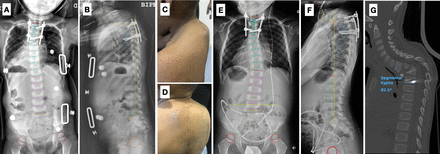
(A and B) Postoperative standing whole-spine radiographs of patient 1, showing T2-T6 posterior instrumentation and a T2-T6 sagittal Cobb angle of 31°. (C and D) Clinical images at 6-month evidencing progression of the upper thoracic kyphosis and implant prominence in the proximal area. (E and F) Six-mo standing whole-spine radiographs evidencing an increase in kyphosis and pull-out of the right cranial screw. (G) Computed tomography scan image corroborating a segmental kyphosis of 82.5°, implant failure, and dislodgement of the anterior bone autograft.
Postoperatively, the patient was immobilized with a cervicothoracolumbar orthosis and a radiological study showed a T2-T6 sagittal Cobb angle of 31°. Mycobacterium tuberculosis was identified by means of polymerase chain reaction (PCR) on tissue specimens, and a histopathologic study revealed chronic granulomatous inflammation with acid-fast bacilli (AFB)-positive staining. One month later, the patient developed severe tuberculous meningoencephalitis (Figure 3A and B), hydrocephalus, and hepatotoxicity secondary to antitubercular drugs, requiring admission to the intensive care unit and a ventriculoperitoneal shunt. On subsequent outpatient visits following recovery, the patient had normal laboratory results but presented gradual progression of the proximal junction kyphosis (Figure 2C–F), with a segmental sagittal Cobb angle of 82° at 6 months and pull-out of the right cranial screw, as well as mobilization of the anterior autograft (Figure 2G); based on these findings, the patient underwent revision surgery. A posterior approach 3-column osteotomy at the apex and circumferential fusion with anterior fibular strut allograft and posterior T1-T7 fixation were executed. After this second intervention, the patient had a reasonably good evolution. He had no neurologic symptoms or evidence of reinfection for the following 3 years, and radiologically, the sagittal Cobb angle was corrected to 25° and maintained during this period (Figure 3C and D). Also, the fibular allograft was incorporated, and there was no evidence of implant failure.
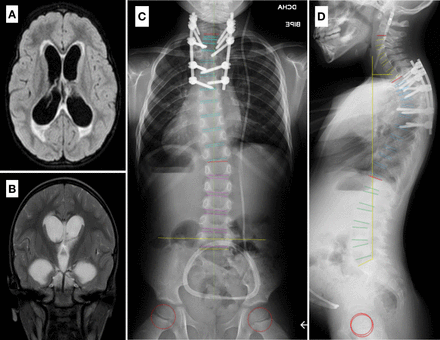
(A and B) Axial and coronal magnetic resonance imaging of the head of patient 1, showing hydrocephalus and tuberculomas. (C and D) Standing whole-spine radiographs of patient 1 taken 3 years after revision surgery, showing T1-T7 posterior instrumentation and acceptable correction of the deformity, with a segmental sagittal Cobb angle of 25°, satisfactory coronal and sagittal balance, and absence of signs of implant failure or nonunion.
PATIENT 2
A 23-month-old Spanish boy was admitted with worsening of his general condition and a 10-day history of limping, which had partially resolved with anti-inflammatory drugs. The parents of the boy recalled no fever or traumatic events. His medical history was unremarkable, and he showed normal psychomotor development. On examination, the child refused to walk, complained of pain when standing, and had a limited left hip flexo-abduction; otherwise, he presented an acceptable general condition and no neurological deficits. Clinical laboratory analysis exhibited a C-reactive protein concentration of 11 mg/dL (0.01–1) and erythrocyte sedimentation rate of 38 mm (1–15).
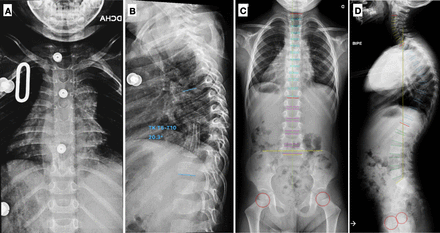
The results of a hip MRI and abdominal ultrasound scan were unremarkable. Nonetheless, a thoracic spine radiograph evidenced angular kyphosis at the midthoracic level of 38° and anterior wedging of the T8 vertebra (Figure 4A and B). Results of an MRI scan were compatible with the presence of a lesion in the T8 vertebral body and T7-T10 anterior paravertebral and intracanal abscesses causing an anteroposterior diameter reduction of the spinal canal (Figure 4C–E). Based on these findings, we suspected that the bone infection was caused by TB. A contact history revealed that 2 people from Central America who lived with the child had developed TB in the previous months and could be the source of the patient’s infection. A Mantoux TST was positive for one of these individuals.
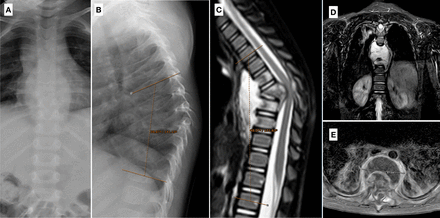
(A and B) Posteroanterior and lateral spine radiograph of patient 2, where signs of a soft-tissue paravertebral abscess can be perceived around the midthoracic spine and angular kyphosis of 38° is present from T5 to T10. (C–E) Magnetic resonance imaging images of the spine evidencing marked kyphosis (48°), T7-T10 prevertebral and intracanal abscesses, and anteroposterior reduction in canal diameter.
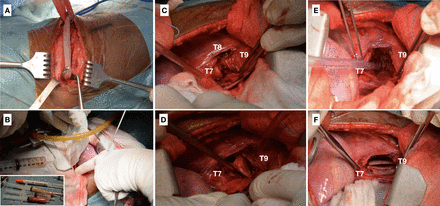
As with the previous patient, a combination of anti-TB therapy and surgery was the chosen course of treatment. After placing the patient in the left lateral decubitus position, a right-sided thoracotomy was performed at the apex; immediately after the rib removal, a pleural bulge and leakage of purulent, caseous material were in evidence (Figure 5). Tissue samples were also sent for culture and histopathologic study. The affected vertebral body was treated from an anterolateral extrapleural approach, removing extensive pathologic tissue and performing spinal cord decompression, followed by debridement of the infected tissue and resection of the remaining T8 vertebral body, thus completing a T8 corpectomy. The resected rib was used to perform an anterior structural autograft to restore sagittal alignment. Due to complete deformity correction and successful spinal stabilization, no posterior instrumentation was added. Neuromonitoring with motor and somatosensory-evoked potentials showed no evident alterations.
Intraoperative images from patient 2. (A) Right thoracotomy at the apex and rib resection. (B) Pleura bulging and opening, with purulent and caseous material drainage and sample extraction. (C) Visualization of T8 vertebral-body destruction. (D) T8 debridement. (E) Removal of devitalized tissues. (F) Placement of the resected rib as an autograft between T7 and T9 for anterior structural support.
The immediate postoperative course was satisfactory, and the patient developed no complications. He was prescribed a thoracolumbar orthosis. His overall general condition improved significantly within a few weeks. Culture results were positive for mycobacteria. Mycobacterium tuberculosis PCR and Mantoux tests were positive, and histology showed necrotizing epithelioid AFB smear-positive osteomyelitis. A routine follow-up examination revealed a favorable evolution. The patient maintained a T5-T10 sagittal Cobb angle of 42° at 4 years, so no additional surgery was required (Figure 6).
(A and B) Postoperative standing spine radiographs of patient 2, showing anterior structural bone graft and angular kyphosis correction without posterior instrumentation with a T5-T10 sagittal Cobb angle of 20°. (C and D) Standing whole-spine posteroanterior and lateral radiographs performed at 4 years postoperatively, evidencing a T5-T10 sagittal Cobb angle of 42°, autograft integration, and an adequate sagittal balance.
Table 1 shows all the relevant information from each of the 2 cases.
Summary of the main characteristics and management of each patient.
DISCUSSION
Two cases of thoracic spinal TB in infants are presented in the current report. Both patients required surgery due to advanced disease, vertebral-body destruction, presence of abscesses, and severe angular local kyphosis (sagittal Cobb angle of over 30°). Clinical presentation and imaging studies are documented. Also, contact studies and laboratory tests, including TST, were carried out to confirm TB diagnosis. Details of the surgical procedures performed consisting of debridement, resection, and reconstruction, as well as all microbiological and histopathologic studies, are also described. Follow-up of each patient was at least 2.5 years.
Although children are more prone to EPTB and more aggressive spinal disease, cases involving very young children are rare and surgical management of very young patients is technically challenging.17 An extensive review of the literature on surgically treated spinal TB cases in patients under 2 years of age revealed only 7 case reports (Table 2). All cases of spinal TB in children younger than 2 years were included in the search. Those lacking detailed individual information about patients, such as the series reported by Mushkin et al7 and Govender et al18 or patients receiving only medical treatment, were excluded.
Summary of the cases of surgically treated spinal TB in patients under 2 years of age reported in the literature.
Among the patients reviewed, the median age was 13 months (range 8–24). There were 5 boys and 2 girls; 5 cases involved the thoracic spine and 2 involved the subaxial cervical spine. These data are consistent with our findings and with worldwide data, which indicate a predominance of male sex and thoracic spine involvement.5 All of the infants reviewed resided in endemic countries (Turkey, China, and India), except for the case described by Consiglieri et al22 in which the patient, like both of our cases, lived in a nonendemic country; however, in all 3 of these cases, the parents had migrated from Central or South America, where TB is prevalent. This fact provides further evidence of the recent increase in TB in developed countries, partly resulting from immigration.3,5
As EPTB has traditionally been considered a disease with numerous manifestations, presenting with varied and generally undefined initial symptoms in patients with spinal TB. diagnosis is usually delayed, as verified in these cases, with a median time from onset to diagnosis of 1 month (range 1–3 months). Unlike data on adults, however, local symptoms such as back or neck pain and difficulty on ambulation, limping, or lower-limb weakness are common and seem to be more evident in this age group. Parental awareness of changes in the child’s behavior or gait pattern likely leads to a more specific identification of symptoms.
When documenting cases, only Cavus et al24 measured preoperative and postoperative kyphosis (82.2° and 11°, respectively), as done in our patients. MRI was also the main imaging technique used to diagnose these toddlers, while a contact study was only performed in a few cases. Both of our patients had MRI scans, which was essential to guide diagnosis, and a contact study revealed close proximity with a TB patient. Clinical, epidemiologic, and radiologic features have been described to be of paramount importance in the early diagnosis of TB.3,11 Also, antitubercular agents were started upon diagnosis in all of the patients, supported by robust evidence that medical therapy is of utmost importance in eradicating TB infection. Mycobacterium tuberculosis PCR or microbiological culture was positive in most of the patients. Histopathologic studies revealed granulomatous lesions with or without caseous necrosis and AFB positiveness.
In most infants, surgical treatment consisted of abscess drainage, debridement and removal of the infected tissues, devitalized bone resection, deformity correction, and spinal reconstruction. Debridement without fusion was performed in 2 patients. The case reported by Tian et al23 was treated by a simple debridement procedure, whereas Lee et al21 also performed a laminectomy. Cavus et al24 described a case of severe iatrogenic kyphosis and neurologic deterioration after previous T3-T4 laminectomies performed at another institution, requiring urgent decompression and posterior stabilization. The rest of the cases were completed with some type of fusion given the importance of achieving stable spinal reconstruction after such an extensive infection causing bone and soft-tissue destruction. Moreover, laminectomy alone without fusion causes further instability and raises the risk of neurologic deterioration. Nonetheless, the 2 infants who received debridement and decompression without fusion did not develop progressive deformity at 24 and 72 months postoperatively.21,23
Classically, the anterior approach has been used to treat infections of the spine, offering direct access to the site of infection, since the anterior portion of the vertebral body is most commonly involved. In addition to enhanced exposure, this approach provides better opportunities for debridement and anterior-cord decompression, as well as more effective support for the anterior column through bone grafting.26,27 Disadvantages are the associated increase in morbidity and progressive kyphosis despite solid anterior fusion due to the immaturity of the pediatric spine and presence of inflamed tissues. In 1997, Schulitz et al28 observed a loss of deformity correction following anterior fusion in children with TB, which could be explained by persistent growth in their posterior spine despite the anterior arrest. To prevent loss of correction, some authors recommend posterior stabilization through pedicle fixation in the same operative setting or at a later stage.29,30 In 3 of the patients reviewed, an anterior approach was taken for debridement and reconstruction using a bone graft. None of the infants had a combined anterior and posterior approach, probably since doing so would have required a second procedure; alternatively, when the procedure is performed in a single stage, operative time and blood loss are greater, causing adding morbidity.27,31 The patient reported by Tufan et al19 was stabilized with an anterior structural graft without instrumentation and developed a progressive increase in the deformity. In our second patient, an anterior graft was applied only, with no posterior instrumentation initially added. This patient also developed some degree of kyphosis during the first postoperative year; however, as no progression was evidenced afterward, no further surgery has been required to date.
Lately, the posterior and posterolateral approach has become increasingly popular, mainly due to their ability to correct kyphotic deformities and achieve spinal stabilization.27 Furthermore, it is less invasive, allows for 360° decompression and resection, and can be more easily prolonged proximally and distally to the involved segment. This approach was performed in the case described by Cavus et al.24 The combination of anterior and posterior reconstruction from a posterior approach has been reported to achieve better results in isolated cases.31,32 Combined reconstruction through a posterior approach was performed in 2 other infants in the series and 1 of our cases, one obtaining anterior fusion by fibular bone grafting,22 another by interbody cage,25 and, in our case, a rib graft. However, some of these cases undergoing combined reconstruction developed progression of the deformity and required revision surgery. In the case reported by Sangondimath et al,25 an anterior cage was combined with posterior stabilization, while in our first case, the rib autograft used for anterior reconstruction was protected with posterior instrumentation. The comparative study performed by Mushkin et al,7 aiming to evaluate the influence of anterior fusion on multilevel reconstruction in young children, concluded that anterior fusion using a titanium mesh cage with bone grafting has advantages over procedures using bone graft only, including accelerated bone fusion and the possibility of earlier posterior instrumentation removal. In most of their patients, posterior instrumentation was also applied, except for 2, due to complete deformity correction and spinal stability achieved through anterior structural grafting only, as was the case in our second patient and the patient reported by Kiymaz et al.20 It should be noted that in both of the cases that required revision surgery, a mechanical cause was evidenced, manifesting as a failure of pedicle-screw purchase in each case, probably due to the reduced bone density of the vertebrae, but without signs of reinfection. Also, the progression of the deformity occurred at the ends of the instrumentation.
Debridement and resection leading to corpectomy were carried out in 2 patients besides ours.20,22 These other patients also underwent anterior reconstruction using a fibular strut allograft, which likely provided sufficient structural support as no deformity was reported during evolution. The literature states that these grafts provide good structural support, though the large quantity of cortical bone makes them less adequate in the presence of infection.33 The iliac crest and ribs are the most common graft types, with the iliac crest providing more support but causing more morbidity compared to rib grafts.34 We decided to use an anterior rib autograft in both of our patients to minimize morbidity; however, this may have provided insufficient anterior support given the progressive deformity that both patients developed. In the revision surgery performed on our first patient, as there was no concern for infection, a fibular bone allograft was used, obtaining better mechanical results.
Single-stage techniques using debridement, grafting, and spinal instrumentation despite active infection have proven to be effective in spinal TB, with satisfactory long-term postoperative outcomes in terms of infection resolution and kyphotic deformity progression.35,36 All of the infants were treated following a single-stage surgical strategy, except for one patient who had undergone previous surgery at another institution,24 and no cases of reinfection affecting the vertebral bodies were documented. Two of the patients developed abscesses that required further surgical drainage. One was located in the psoas muscle19 and another in the cervical spine, which caused airway obstruction,21 a relatively common complication when EPTB involves this region.37 In the cases reviewed, decompression of the neurological structures was performed in the presence of neurological symptoms or evidence of spinal cord compression. The median follow-up time was 24 months (range 12–72), with our patients receiving the second-longest monitoring after surgery.
The revision surgery performed on our first patient consisted of a single-stage posterior approach for 3-column osteotomy at the apex with additional anterior fibular allograft support and enhanced posterior pedicle-screw instrumentation to restore sagittal spine alignment and achieve circumferential stabilization. Sangondimath et al25 described a similarly complex procedure involving a posterior approach in a single surgical session in a 2-year-old boy with spinal TB. The authors performed a single-stage posterior vertebral column resection (VCR) at T4-T7 followed by placement of an anterior cage and posterior pedicle-screw instrumentation. Rangel-Castilla et al38 performed a VCR using bilateral costotransversectomy with an anterior expandable titanium cage in a 3-year-old girl and achieved 360° stabilization using posterior pedicle instrumentation with reconstruction of the kyphotic deformity. Chang et al39 compared the long-term outcomes of posterior VCR for congenital scoliosis in children who had surgery before 10 years of age with those over 10 years. They recommended that fusion should be as short as possible but must be selected based on criteria to achieve deformity correction, prevent progression, and avoid influencing adjacent vertebral growth. The authors also noted that 1- or 2-level fixation was sufficient in children under 6 years of age, that is, before structural changes; otherwise, they recommended fusion of more than 2 or 3 levels above and below the infection site.
To our knowledge, this is the first report describing surgical treatment of spinal TB in 2 consecutive patients under 2 years of age, with a thorough description of the cases, including clinical, laboratory, and radiological findings, detailed surgical treatment, microbiologic and histopathologic results, and medium-term follow-up. Some interesting conclusions can be established after reviewing the cases of surgically treated spinal TB in infants. These include a greater prevalence among men, more frequent involvement of the thoracic region, presentation in patients who typically reside in epidemic countries or in children of immigrant parents, a median diagnostic delay of 1 month, the importance of MRI as a guide for diagnosis, and contact study to narrow the differential diagnosis. Early diagnosis and medical treatment are fundamental to inactivate the infection, but in the event of advanced-stage spinal TB, vertebral collapse, severe deformity, abscesses, or neurological compromise, appropriate surgical treatment is indicated. Though challenging in infants, spine surgery has been shown to be an aid in eradicating the infection, decompression to relieve neurological symptoms, correction of the spinal deformity, and reconstruction to restore spinal balance and avoid progression. When treating patients with extensive vertebral-body destruction or severe kyphosis, solid anterior support and sufficiently long posterior instrumentation may achieve better outcomes, minimizing the risk of further deformity. As the number of patients reviewed is small and the surgical strategies are heterogeneous, additional studies with more extensive patient series, uniform surgical management, more accurate outcome measurements, and longer follow-up periods are required to reach a consensus on this complex issue.
CONCLUSION
Surgical treatment is indicated in the presence of vertebral collapse, severe deformity, abscesses, or neurological compromise. Solid anterior support and adequate posterior instrumentation are necessary to prevent deformity progression when a patient presents extensive vertebral-body destruction or severe kyphosis, even at very young ages.
Footnotes
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Conflicting Interests The authors report no conflicts of interest related to this work.
Disclosure The authors report no financial disclosures related to this article.
Ethics approval This study was carried out in accordance with the World Medical Association Declaration of Helsinki (JBJS 79A:1089-98,1997).
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2022 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.