Abstract
Historically, thoracic disc pathology has been treated via open thoracotomy or open posterior costotransversectomy or lateral extracavitary approaches. However, these approaches are associated with approach-related morbidity. With advancement in such minimally invasive approaches as the lateral interbody fusion coupled with navigation, the morbidity of approaching anterior thoracic spinal pathology may be reduced. There are subtleties and nuances in the thoracic approaches that are different from the lateral lumbar interbody approaches. We discuss our technique of the minimally invasive approach to the thoracic spine, management of the rib and pleura, and incorporation of navigation into the procedure.
- thoracic disc
- lateral surgery
- retropleural approach
- minimally invasive surgery
- lateral
- thoracic fusion
- lateral thoracic discectomy
INTRODUCTION
Although the lateral access for thoracic disc pathology has been present for decades, the standard approaches have historically been the open thoracotomy or the extracavitary approach. Both of these approaches carry significant morbidity.1,2 A mean complication rate of 39% for thoracotomy and 17% for lateral extra-cavitary is reported in the literature.3 The costotransversectomy and transpedicular approaches have also historically been used, but these approaches have limited access to the direct ventral aspect of the spine and to the contralateral side of the spinal column. The lateral approach to the thoracic spine not only incorporates the advantages of the thoracotomy, allowing for complete, bilateral access to the anterior thoracic spinal canal, but also incorporates a minimally invasive aspect to the surgery, decreasing morbidity.
The lateral approach was initially developed for the lumbar spine,4,5 but the concept has been used for thoracic spine pathology.6 Increasingly, reports of outcomes have been published, and the feasibility of the minimally invasive lateral approach to the thoracic spine has been established. We discuss the technique of the lateral approach to the thoracic spine, its challenges, and outcomes.
HISTORY AND DEVELOPMENT
The true incidence of herniated thoracic discs is unknown since many cases go unrecognized, and only a handful of patients are symptomatic. The majority of patients are identified in the fourth through sixth decades of life.7–10 In a review of 288 cases reported in the literature, Arce and Dohrmann9 noted a slight male preponderance (1.5:1); however, most series show a nearly even gender distribution.11–14 The major contributory factor to thoracic disc herniations is thought to be a degenerative process.7,14,15 This theory is supported by the higher incidence of herniations in the thoracolumbar spine, where the greatest degenerative changes occur.15–17 The identification of trauma as the cause of herniated thoracic disc is controversial because a history of trauma can only be elicited in 14% to 63% of patients.10,17 One major coindicator of thoracic disc herniation in Scheuermann’s disease.18,19 Most cases of disc herniations occur between T8 and L1, with 26% to 50% of these cases occurring at T11/12.15,19
A multitude of surgical options have resulted in various types of surgical exposures to treat different morphologies of thoracic disc herniations. Historically, surgical treatments had been posterior approaches via decompressive laminectomy.20 However, these treatments were associated with a high complication rate. A review of the literature by Perot and Munro in 1969 recorded 91 patients with thoracic disc herniations who underwent decompressive laminectomies. Of those, only 56% experienced either partial or complete symptom resolution, with 18% being rendered paraplegic and 7% dead.20
Some consider the anterior transthoracic approach to be the gold standard in terms of visualization in the thoracic spine20–23 since it avoids the mobilization of spinal cord, which may need to be performed from the posterior approach.7,12,20,24,25 However, there is an associated morbidity with thoracotomy. The thoracotomy complication rate is reported to range from 39% to 100%,3,26 with the most common complications being pneumonia, pneumothorax, atelectasis, and post-thoracotomy pain in up to 30% of patients at 5-years postoperative.27 Furthermore, open thoracotomy is associated with longer length of stay12,22,28 and increased blood loss.22,29 The posterolateral approach eliminates the need for cord retraction, except in cases of far contralateral herniations. However, the working field is limited (especially in minimally invasive exposures) and typically involves extensive bony resection and rhizotomy of the ipsilateral nerve root.1,2,12,22,27–39
ADVANCES AND MODERN OUTCOMES
Minimally Invasive Spine Surgery
The mini-open lateral approach utilizes direct visualization and an adequate working field for thoracic disc pathology; it does not require single-lung ventilation. The working field, while smaller than that for open thoracotomy, has the advantage of surgical manipulation without a fixed point, unlike thoracoscopic approaches, which are limited by the working ports that are fixed in space by the chest wall. In a multicenter study by Uribe et al,40 60 consecutive patients underwent lateral interbody fusion (LIF) for thoracic disc pathology with an average follow-up of 11 months. Median operative time was 182 minutes, average blood loss was 290 mL, and average hospital stay was 5 days. Minor complications occurred in 7% of patients. Four patients had major complications including pneumonia, extra-pleural free air, new lower extremity weakness, and posterior wound infection. At last follow-up, the visual analog scale (VAS) improved 60% (7.8–3.1).40 In their study, symptoms substantially improved: myelopathy improved in 83%, radiculopathy improved in 87%, and back pain improved in 91% of cases. At last follow-up, 80% of patients experienced “excellent” or “good” outcomes, with 15% exhibiting “fair” or “unchanged” outcomes, and only 5% experiencing “poor” outcomes.40 This study suggests that the minimally invasive lateral approach is a reasonable procedure for achieving adequate decompression of thoracic disc herniations in a less invasive manner without the use of thoracoscopy.40
TECHNIQUE
Navigation and Incision Planning
The use of navigation has become common in spine surgery, but it also has an increased role in lateral lumbar spine surgery.41,42 The advantages of navigation are numerous. The improved accuracy, the obviation of lead aprons, and the elimination of continual fluoroscopy during the case are some positive aspects of navigation in the spine. However, there are some specific advantages with regard to the use of navigation during lateral thoracic surgery.
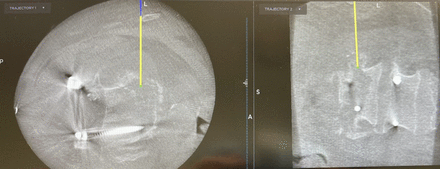
The lateral fusion surgery is a minimally invasive spine (MIS) procedure, and because of such a small, focal incision, the planning of the incision over the correct interspace and at the correct orientation is critical. With the open thoracotomy, the thoracic level of interested was noted preoperatively, and the thoracotomy was usually performed at the ribs 1 or 2 levels above the thoracic pathology because of the down sloping ribs. This usually resulted in a reasonable approximation of exposure to the thoracic spine but may not always have been the ideal trajectory. With navigation, the ideal trajectory can be planned before the skin incision is made (Figure 1). We currently register the navigation with the reference arc in the iliac crest (Figure 2), and we localize the incision over the thoracic spine. This allows the MIS incision to be made exactly over the pathologic level and the correct angle. The navigational probe projection can be extended down to the pathologic level, ensuring proper incision placement and facilitating the MIS approach.
Using navigation, the correct level and the overlying rib that needs to be resected in order to obtain the ideal trajectory are identified. This localization allows for precise incision planning in setting of minimally invasive surgery.
The reference arc is placed in the iliac crest to allow for registration navigation system.
There are other advantages of navigation that are specific to the thoracic spine. One advantage of navigation is that it allows the surgeon to clearly identify the position of the aorta and vena cava. Because the thoracic vertebrae are smaller and triangular shaped, the aorta can be immediately adjacent to the left side of thoracic vertebrae. Knowledge of the aortic position is critical in order to avoid injury or impingement when placing instrumentation from the contralateral side.43 The aortic position relative to the vertebral body can be even more critical in the upper thoracic spine when the aorta is lateral, not anterior, to the spine and in scoliosis cases. The axial rotation during scoliosis cases creates anatomic abnormalities, and the position of the aorta relative to the spine is important. The other important advantage that navigation confers is clear identification of the spinal canal, which again can be critical in scoliosis cases. Historically in anterior thoracic approaches, identification of the rib head, pedicle, and neural foramen has been used to identify the spinal canal. However, these anatomic landmarks are subject to degeneration, axial rotation, and inaccurate anatomy in cases of pathologic destruction. The use of navigation allows the surgeon to immediately identify the spinal canal and the correct level before any bony work on the anterior thoracic spine has been begun. This affords a safe and accurate trajectory to either avoid the spinal canal in cases of fusion only or safely decompress the spinal canal in cases that need decompression. Finally, because the navigation is based on a computed tomography (CT)-type image acquisition, the localization of the correct pathologic level can be more accurate than plain intraoperative radiographs or fluoroscopy. Moreover, on some navigational platforms, preoperative magnetic resonance images may be fused with intraoperative CT-type imaging to further aid in proper localization. Pathologic vertebral body destruction, identifying osteophytes or unique anatomic features, and even preoperatively placed fiducial markers or methylmethacrylate can be easily identified with navigation, ensuring correct-level surgery. Thus, we have found many advantages with the use of navigation to make the surgery more accurate and safer, especially in cases of spinal deformity or anatomic abnormalities.
Management of the Rib and Pleura
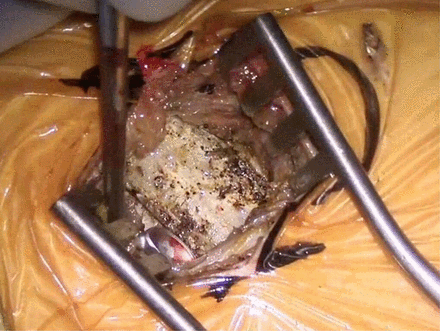
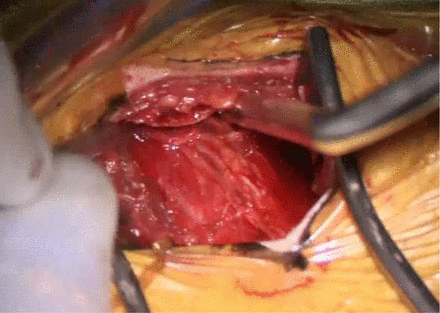
As general rule, the retropleural approach to the thoracic spine is ideal because it avoid the use of a chest tube, dual lung ventilation, risk of injury to the lung, and the need of an access surgeon. However, even with the most meticulous of dissection, there are often times pleural rents. One way to mitigate the risk of pleural violation is to actually resect a small piece of rib for the access (Figure 3). By resecting a small piece of rib, the retropleural plane is first identified, and the plane can be expanded dissection against the dorsal aspect of the ribcage toward the spine. This allows a ventral displacement of the pleura off the ribcage (Figure 4). In cases where pleural rents occur, the most important concept is to evacuate the pleural air, which does not necessitate placement of a formal chest tube. Just as interventional radiology uses a pigtail catheter for pneumothoraces, a small pleural drain to evacuate the air postoperatively usually suffices. Although various drains can be used, we use a 19-French channel drain that is threaded up toward the apex of the lung, and we ensure that anesthesia does not allow the patient to self-ventilate until the wound is closed, maintaining positive pressure to evacuate out any pleural air. We usually place the drain right before closure of the chest wound, after the thoracic spine surgery has been completed. The drain is tunneled out through a separate stab incision in order to prevent a pneumothorax when the drain is pulled. The drain is kept on suction, and a formal chest tube apparatus is generally not needed; once the drain output is less than 250 cc in 24 h and there is no significant pneumothorax on chest radiographs, the drain is pulled. After the drain has been pulled, a chest radiograph to ensure there is no pneumothorax is also obtained. One way to evacuate intrapleural air without leaving the catheter is to have the anesthesiologist provide continuous positive pressure ventilation with a catheter in the intrapleural space. Next, the anesthesiologist is asked to perform a Valsalva maneuver, which should squeeze most of the air out of the chest while the catheter is left in place. After the air is evacuated, and the anesthesiologist continues to hold the Valsalva, the catheter is removed, hopefully leaving the intrapleural space devoid of the majority of air.
The correct rib is identified and dissected via electrocautery. A short segment of rib is resected to allow for easier retropleural dissection.
After rib resection, the retropleural space is identified, and the dorsal aspect of the rib is followed to reach to the spine.
As the dissection path continues toward the spine, the rib head can be identified. The rib head usually covers the pedicle and part of the disc space. In order to fully gain access to the thoracic spinal canal, the rib head is usually resected using a high-speed bur to first transect the rib head from the remainder of the rib, and the rib head can be cracked and separated with an osteotome. We prefer not to use the osteotome with a mallet against the rib head itself in order to prevent inaccuracy of the navigation, but certainly this is an expeditious option to remove the rib head.
Management of the Diaphragm
In the lower thoracic spine, the diaphragm will often be in the way of a direct approach to the spine. Generally, the diaphragm can be gently pushed ventrally and inferiorly during the blunt dissection and approach. However, there are times when the T12-L1 level needs to be accessed. Dakwar et al have opined that a minor diaphragmatic detachment during the minimally invasive approach does not have clinical sequelae.44 In our practice, we do not detach the diaphragmatic attachments, but rather, we split the muscle in the direction of its fibers, just as the psoas muscle is dissected in the direction of its fibers, not necessarily detached. We have found that the small dissection of the diaphragm in the direction of its fibers facilitates access to the T12-L1 level. Similarly to the experience of Dakwar et al, the opening is quite small, and direct closure usually is not performed as we have not found clinical sequelae from this method of diaphragm dissection.
Placement of Minimally Invasive Retractor, Disc Removal, and Fusion
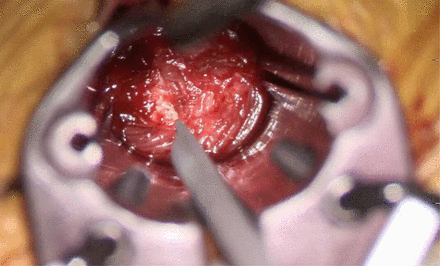
After the disc space has been identified (Figure 5), a navigated probe, which is also the first and smallest dilator, is placed into the disc space under navigation. The correct orientation and depth are verified using navigation. The subsequent dilators are placed, and a standard minimally invasive retractor is placed retropleurally in a true perpendicular orientation to the spinal column. For interbody fusion procedures, the disc is incised (Figure 6), and standard lateral lumbar interbody fusion instruments are used to prepare the endplates and size the implant. Because these instruments are navigated, very accurate trajectories away from the spinal canal and aorta can be achieved without the use of fluoroscopy. After the endplates have been prepared, the interbody graft can be placed also under navigation in an accurate manner. We usually confirm placement of the graft using fluoroscopy in the event that there is any shift in the navigation during the operation.
The appropriate disc space is identified, and the level is verified with navigation.
The minimally invasive retractors are placed to retract the pleura and allow for disc removal in the standard fashion.
If a decompression needs to be performed by removal of a midline thoracic disc herniation, we generally begin by identifying the spinal canal using navigation. If navigation is not available, the spinal canal can be identified using a blunt probe just below the pedicle to palpate the neural foramen after removal of the rib head. Once the spinal canal has been identified, part of the vertebral body above and below the disc space is removed using a high-speed bur. The intervening disc is then removed using a pituitary rongeur, resulting in a cavity with which to ventrally remove disc material. In order to carefully identify the posterior longitudinal ligament and dura, the microscope is used for clear visualization. The posterior vertebral body wall is carefully drilled until a cavity is created above and below the pathologic disc. The spinal cord is then identified cephalad and caudad to the disc. Once the plane between the dura and the posterior longitudinal ligament has been identified, the ventral pathology can be bluntly dissected away from the dura. Subsequently, using blunt straight curettes, the thoracic disc can be slowly and methodically pushed ventrally away from the spinal cord into the cavity previously created. This step is repeated until the entire spinal cord is decompressed all the way to the contralateral pedicle, which can be either identified with navigation or directly palpated using a blunt Penfield instrument. Although placing an interbody cage can be performed, we generally do not place a cage after a thoracic discectomy because of the risk of posterior displacement of the cage into the spinal canal. Arthrodesis is performed using the morselized rib head gently placed into the interspace after decompression, away from the spinal canal. If necessary, instrumentation can be performed with either anterior fixation or posterior instrumentation, but usually, the spine is stable enough not to warrant any instrumentation. For closure, a drain is left in place if hemostasis cannot be adequately achieved, and the wound is closed in layers, being careful not to strangulate the intercostal nerves using the suture in order to avoid postoperative radiculopathy (Figures 7 and 8). Because of the mini-open nature of this approach, traditional pericostal sutures are not placed, and this helps obviate the post-thoracotomy pain by not placing tension on the intercostal nerves.
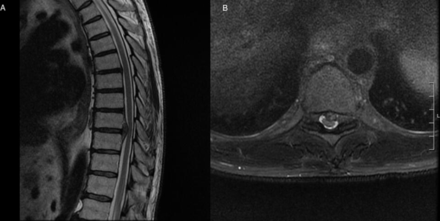
A preoperative T2-weighted magnetic resonance imaging showing a large paracentral disc protrusion at T9-10 with cord indentation.
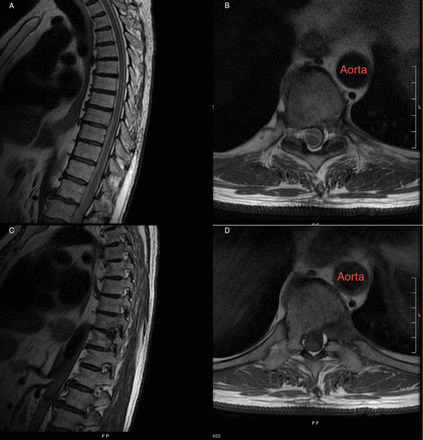
A postoperative T2-weighted magnetic resonance imaging (MRI) with sagittal (A) and axial views (B) showing decompression of the spinal cord after disc excision and a T1-weighted MRI (C+D) showing the T10 rib head resection and left-sided T10 pediculectomy.
OUTCOME DATA
With regard to thoracic interbody fusion, Karikari et al did a retrospective review of 22 patients who underwent LIF at the thoracic spine and the thoracolumbar junction.45 The mean VAS for lower extremity pain improved from 7.3 preoperatively to 4.6 postoperatively. The mean preoperative and postoperative Oswestry Disability Index scores were 42 and 34, respectively. In patients treated for degenerative scoliosis, the average improvement was 8° both in coronal (0°–25°) and in sagittal planes (0°–20°).
With regard to thoracic disc herniations, Nacar et al reported a series of 33 consecutive patients with thoracic disc herniations who underwent anterior spinal decompression followed by instrumented fusion through a lateral approach. Patients were followed-up for an average of 18.2 months, and the mean length of hospital stay was 5 days. Six patients developed complications including cerebrospinal fluid leak, intercostal neuralgia, urinary retention, ileus, and pleural effusion with the need for chest tube insertion.46 All complication-related symptoms resolved at the last follow-up visit. Myelopathy improved in 91% of patients. The mean preoperative VAS pain scores, Oswestry Disability Index scores, and Short Form Survey-36 physical and mental component summary scores had all improved.46 In another study performed by Oltulu et al, 59 patients underwent minimally invasive lateral thoracic disc excision and fusion with a mean follow-up of 60 months.47 Thirty-two patients had calcified disc herniations, and 90.7% of patients with myelopathy had improvement in their neurological status postoperatively.
Von Glinski et al reviewed the risk profile of the lateral approach to the spine at different regions. It was found that the highest rates of neurological complications (42.9%) (sensory only) and instrumentation failure (14.3%) after 6 months were in the thoracic spine.48 The highest rate of pleural violation (13.6%) occurred at the thoracolumbar junction. When an adjustment for corpectomy was done, there was not any significant difference in the risk among the thoracic, thoracolumbar, and lumbar regions.48
There is no consensus regarding fusion after thoracic discectomy. General indications for fusion include kyphosis, scoliosis, severe mechanical back pain, extensive bony resection of the vertebral body or posterior elements, multiple thoracic discectomies, or the presence of Scheuermann’s disease.18,19 Previous studies have shown that instrumented fusion is usually not necessary with standard thoracoscopic or transthoracic discectomies, which involve the removal of the ipsilateral rib head, ipsilateral pedicle, and a portion of the posterior vertebral body.49–53 Only about 1.8% to 10.1% of patients develop postoperative spinal instability.52,54 Instrumented fusion is generally needed when there is resection of about 50% of the vertebral body resected.
CONCLUSION
When using the anterior approach to the thoracic spine, the minimally invasive lateral approach is a viable option. A minimally invasive or mini-open approach allows the surgeons to have increased instrument mobility compared to thoracoscopy. The use of navigation can allow a more accurate, focused approach to the thoracic spine and potentially allow for complication avoidance by accurate localization of critical structures and by orientation of the spine in deformity cases. The addition of the microscope allows for a safe dissection of the thoracic pathology away from the dura and spinal cord. With the mini-open approach to the spine, the morbidity of a formal thoracotomy can be decreased with the added advantage of direct access to anterior pathology.
Footnotes
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests Nitin Agarwal has received royalties from Thieme Medical Publishers. Dean Chou has served as a consultant for Globus. Praveen V. Mummaneni has served as a consultant for DePuy Spine, Globus, and Stryker; has direct stock ownership in Spinicity/ISD; has received royalties from DePuy Spine, Thieme Publishers, and Springer Publishing; and has received support from ISSG, NREF, and AOSpine for non–study-related clinical or research effort. The remaining authors have no disclosures.
Disclosure The authors report no financial disclosures related to this article.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2022 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.