Abstract
Background Over the past 20 years, multiple randomized controlled trials have shown cervical disc arthroplasty (CDA) to be safe and effective for treating 1- and 2-level degenerative disc disease (DDD). The purpose of this postmarket study is to compare 10-year outcomes between CDA and anterior cervical discectomy and fusion (ACDF) from a randomized study at 3 centers.
Methods This study was a continuation of a randomized, prospective, multicenter clinical trial comparing CDA with the Mobi-C cervical disc (Zimmer Biomet) vs ACDF. Following completion of the 7-year US Food and Drug Administration study, 10-year follow-up was obtained from consenting patients at 3 high-enrolling centers. The clinical and radiographic endpoints collected at 10 years included composite success, Neck Disability Index, neck and arm pain, short form-12, patient satisfaction, adjacent-segment pathology, major complications, and subsequent surgery.
Results A total of 155 patients were enrolled (105 CDA; 50 ACDF). Follow-up was obtained from 78.1% of patients eligible after 7 years. At 10 years, CDA demonstrated superiority to ACDF. Composite success was 62.4% in CDA and 22.2% in ACDF (P < 0.0001). The cumulative risk of subsequent surgery at 10 years was 7.2% vs 25.5% (P = .001), and the risk of adjacent-level surgery was 3.1% vs 20.5% (P = .0005) in CDA vs ACDF, respectively. The progression to radiographically significant adjacent-segment pathology at 10 years was lower in CDA vs ACDF (12.9% vs 39.3%; P = 0.006). At 10 years, patient-reported outcomes and change from baseline were generally better in CDA patients. A higher percentage of CDA patients reported they were “very satisfied” at 10 years (98.7% vs 88.9%; P = 0.05).
Conclusions In this postmarket study, CDA was superior to ACDF for treating symptomatic cervical DDD. CDA was statistically superior to ACDF for clinical success, subsequent surgery, and neurologic success. Results through 10 years demonstrate that CDA continues to be a safe and effective surgical alternative to fusion.
Clinical Relevance The results of this study support the long-term safety and effectiveness of cervical disc arthroplasty with the Mobi-C.
Level of Evidence 1.
- cervical disc arthroplasty
- Mobi-C
- degenerative disc disease
- adjacent-segment pathology
- anterior cervical discectomy and fusion
INTRODUCTION
Anterior cervical discectomy and fusion (ACDF) has been the standard surgical treatment for symptomatic cervical spondylosis since the mid-20th century. In the past 20 years, multiple randomized controlled trials (RCTs) have shown cervical disc arthroplasty (CDA) to be safe and effective for the treatment of both 1- and 2-level cervical degenerative disc disease (DDD). ACDF is known to alter segmental motion at adjacent vertebrae, which places additional stress on adjacent discs that may accelerate degeneration.1–3 By preserving spinal motion, CDA may reduce degeneration at the adjacent segments compared with ACDF.3–8
The US Food and Drug Administration (FDA) approved the Mobi-C cervical disc (Zimmer Biomet [now ZimVie], Westminster, CO) in 2013 for treatment of 1- or 2-level cervical DDD with radiculopathy and/or myelopathy. Upon approval, the FDA required a postapproval study to collect data out to 7 years. The Mobi-C was shown statistically superior to ACDF in terms of composite measures of overall success through 7 years.9–13 The safety and effectiveness have recently been reported out to 10 years in a cohort from the FDA study.14 The purpose of this postmarket study is to compare 10-year outcomes between CDA and ACDF from a randomized study at 3 centers.
METHODS
Study Design
This was a prospective, randomized study of patients treated with CDA or ACDF at 3 centers. Patients were enrolled in the prospective, randomized multicenter investigational device exemption (IDE) clinical trial (ClinicalTrials.gov registration no. NCT00389597). Institutional review board approval and patient informed consent were obtained at each investigational site. The study was divided into separate arms of 1- and 2-level treatment, conducted in tandem. Enrollment criteria included a diagnosis of DDD with radiculopathy or myeloradiculopathy at either 1 or 2 contiguous levels from C3 to C7, with no prior cervical operations. The details of the study protocol, inclusion and exclusion criteria, and patient characteristics have been reported previously.13
Patient Selection
Surgeries occurred between May 2006 and March 2008. The investigational group was treated with 1- or 2-level CDA. The control group received 1- or 2-level ACDF with allograft and anterior cervical plate. Patients were randomized in a 2:1 ratio (CDA:ACDF). Upon completion of the 7-year FDA postapproval study, 3 high-enrolling centers committed to continue follow-up beyond the 7-year follow-up required by the FDA. These centers collaborated to obtain follow-up at 10 years for consenting CDA and ACDF patients. The patients from the 3 centers accounted for more than 25% of the original IDE cohort.
Study Device
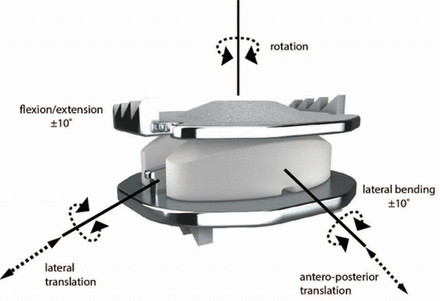
The Mobi-C is a 3-component, mobile-bearing device comprised of an ultra-high-molecular-weight polyethylene mobile insert between 2 titanium plasma-sprayed and hydroxyapatite-coated cobalt-chromium-alloy endplates. The superior endplate incorporates a convex shape to match the natural cervical anatomy, and both the superior and inferior endplates feature low profile, inclined teeth along the lateral edges to provide initial stability. The shape of the device and inclined teeth were designed to facilitate a bone-sparing surgical technique. The device is available in several footprints and a range of heights, including 5 mm, to accommodate individual anatomical requirements. The device allows 5 independent degrees of freedom: 2 translational and 3 rotational (Figure 1).
The Mobi-C cervical disc.
Outcomes
The outcome measures were defined in the original IDE study and included secondary surgical procedures (removals, revisions, reoperations, or additional fixation), adverse events (AEs), the Neck Disability Index (NDI), visual analog scale neck and arm pain, short form-12 (SF-12) physical component score (PCS) and mental component score (MCS), patient satisfaction, and neurologic function. The neurologic function was assessed with tests of sensory, reflex, and motor function. Neurologic success was defined as maintained or improved motor, sensory, and reflex assessment compared with preoperative baseline. Radiographic adjacent segment pathology (RASP) was defined with the Kellgren-Lawrence Scale.15 Grade 3/4 RASP was considered radiographically significant. Independent radiologists (Medical Metrics, Inc., Houston, TX) conducted radiographic evaluations.
Similar to the IDE study, a composite endpoint was used to define clinical success. A patient was considered to have a successful outcome at 10 years if each of the following criteria were met:
The NDI score improved by at least 15/100 points for a patient with a preoperative NDI score of 30 or greater or improved by at least 50% of a preoperative NDI score of less than 30.
No subsequent surgical intervention occurred at the index or adjacent level(s).
No serious treatment-related AEs occurred.
Neurologic function was not worse than the preoperative function.
Patients with a subsequent surgery or treatment-related AE at any timepoint were carried forward as a failure in the 10-year success endpoint.
Statistical Analysis
All patients and follow-up from 3 sites were included in the analysis. All 1- and 2-level patients were pooled for this analysis due to the low number available at 10 years, especially in the ACDF cohort. The baseline characteristics between the cohort from the 3 centers and the remaining patients from the IDE trial were compared to show that this subset was representative of the original FDA study cohort.
The composite success endpoint was assessed under the hypothesis of noninferiority of CDA vs ACDF using the Farrington-Manning test with a 10% noninferiority margin. Noninferiority was defined using a 95% 1-sided lower confidence bound of −10% for the difference between CDA and ACDF, and superiority was tested using a lower confidence bound of 0%. Repeated measures mixed effects analysis of variance was used to compare postoperative outcomes between CDA and ACDF patients, as well as to compare 10-year results with preoperative and 7-year outcomes within the CDA and ACDF groups. P values and confidence limits were adjusted for multiplicity using a Monte Carlo simulation-based method. Survival function estimates for secondary surgery and device-related AEs were calculated using the Kaplan-Meier method, with the log-rank test to compare survival functions. All patients who were withdrawn or lost to follow-up were censored at their last visit prior to study withdrawal. Categorical proportions were compared using Fisher’s exact test for independent samples. Confidence intervals for proportions were calculated with the Clopper-Pearson exact binomial method. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
The sensitivity analysis was performed to investigate the potential impact of dropouts on overall study success and rate of subsequent surgery at 10 years. Each dropout was considered a hypothetical success or failure to investigate the effects of all possible combinations of the values of missing data in the CDA (treatment) vs the ACDF (control) group. The sensitivity analysis was run against all combinations of success and failure to determine the tipping point of the study (ie, the combinations of success and failure among dropouts that would change the study conclusions).
RESULTS
Study Cohort
The original enrollment at 3 sites was 155 patients (105 CDA; 50 ACDF). There were no significant differences in preoperative and operative characteristics between the CDA and ACDF treatment groups (Table 1). The comparison of baseline characteristics between patients from the 3 centers and the remaining patients from the IDE trial found no significant differences in preoperative characteristics between these patients and the original FDA cohort. Outcomes at 7 years for the 3-center cohort were also similar to those reported for the IDE study.
Preoperative and operative characteristics of CDA and ACDF patients at 3 centers.
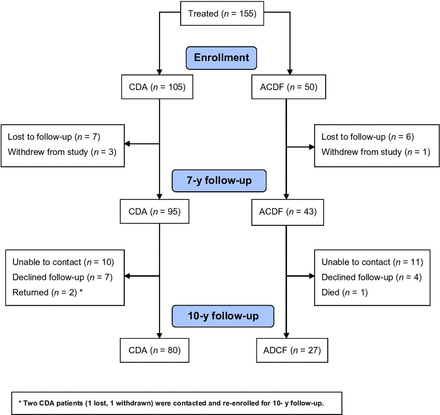
Follow-up was obtained from 107 patients at 10 years, representing 69% (107/155) of all patients enrolled at these sites, and 78.1% (107/137) of patients available after 7 years, after excluding 1 patient who died prior to 10 years (Figure 2). Five patients who did not return for in-person follow-up did not have 10-year radiographs, but patient-reported outcomes (NDI, pain, and SF-12), AEs, and reoperation were collected via phone interview and review of medical records. The longest follow-up was 13.1 years.
Flow diagram of patient enrollment and follow-up. ACDF, anterior cervical discectomy and fusion; CDA, cervical disc arthroplasty.
Composite Success
At 10 years, CDA demonstrated superiority compared with ACDF (Table 2). The rate of success was 62.4% (53/85) in the CDA group and 22.2% (8/36) in the ACDF group, with a difference of 40.1% and a lower 95% confidence bound of 23.9%. Analyzing the individual components of success showed that the primary drivers of CDA superiority were a significantly lower incidence of subsequent surgery and a higher incidence of neurologic success.
Overall success and components of success for CDA and ACDF at 10 years.
Sensitivity Analysis
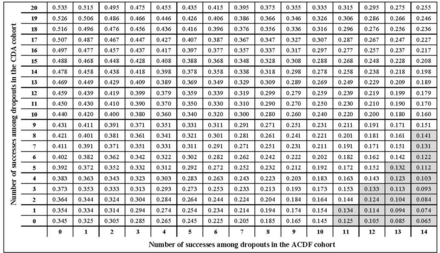
At 10 years, there were 20 CDA and 14 ACDF dropouts for whom composite success and subsequent surgery were unknown. The sensitivity analysis evaluated all 315 combinations of success and failure. For composite success, the sensitivity analysis supported the study conclusion of superiority of CDA vs ACDF in 93.3% of the scenarios (Figure 3). Therefore, when we consider all possible outcomes among dropouts, we conclude there is only a 6.7% chance of obtaining a different conclusion. The tipping point for study success occurred if at least 79% (11 of 14) of ACDF dropouts were a study success, combined with study failure in 95% (19 of 20) of CDA dropouts. Although CDA was not statistically superior to ACDF in those cases, the observed treatment effect was always positive, and no scenario resulted in the superiority of ACDF over CDA. Under the worst-case scenario (all ACDF dropouts = success; all CDA dropouts = failures), CDA was shown to be noninferior to ADCF (50.5% vs 44%; Δ = 6.5%; P = 0.027).
Tipping point analysis for composite success showing estimated treatment effect (difference between cervical disc arthroplasty [CDA] success and anterior cervical discectomy and fusion [ACDF] success). Axes represent the number of successes that could be observed among dropouts in the CDA group and the ACDF control group. White cells indicate superiority of CDA vs ACDF, and gray indicates noninferiority for each combination of successes. The lower right corner represents the worst-case scenario (all ACDF missing = success; all CDA missing = failures).
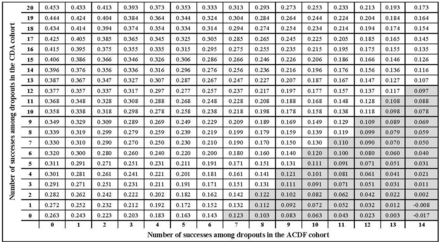
For subsequent surgery, 81.3% of the scenarios in the sensitivity analysis supported the conclusion of superiority of CDA vs ACDF (Figure 4). Therefore, the chance of obtaining a different conclusion for subsequent surgery is 18.7%. The tipping point for study success occurred if at least 50% of ACDF dropouts were a study success, combined with study failure in 100% of CDA dropouts. In those cases, CDA was not superior to ACDF, but no scenario resulted in superiority of ACDF over CDA.
Tipping point analysis for subsequent surgery showing estimated treatment effect (difference between cervical disc arthroplasty [CDA] surgery and anterior cervical discectomy and fusion [ACDF] surgery). Axes represent the number of successes that could be observed among dropouts in the CDA group and the ACDF control group. White cells indicate superiority of CDA vs ACDF, and gray cells show where CDA was not superior to ACDF. The lower right corner represents the worst-case scenario (all ACDF missing = no surgery; all CDA missing = surgery).
Safety
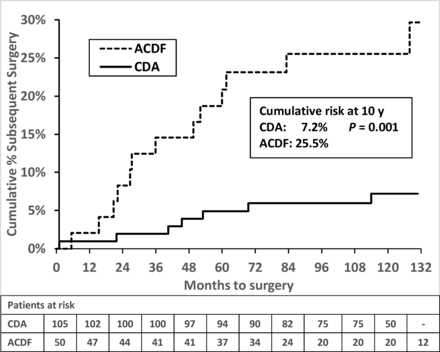
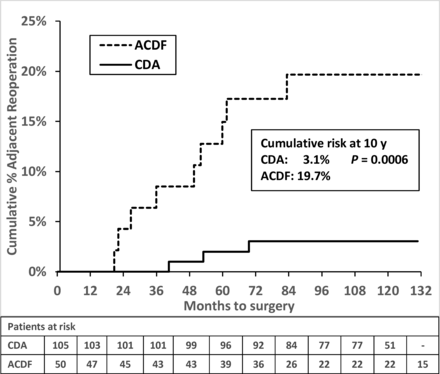
After 7 years, 1 CDA patient underwent supplemental fixation at the index level, 9.5 years after surgery. One ACDF patient had nonadjacent fusion 10.6 years after surgery, followed by an adjacent-level fusion 11.7 years after surgery. There were no adjacent-level surgeries reported in CDA patients after 7 years. The cumulative risk of any subsequent surgery at 10 years was 7.2% vs 25.5% (P = 0.001) in CDA vs ACDF (Table 3; Figure 5). The cumulative risk of an adjacent-level surgery was 3.1% vs 20.5% (P = 0.0005) in CDA vs ACDF, respectively (Table 3; Figure 6). All cases of adjacent-level surgery in this series were due to symptomatic adjacent-level disease. The risk of adjacent surgery in the CDA cohort was unchanged after 6 years.
Cumulative risk of subsequent surgery and treatment-related adverse events at 10 y after CDA or ACDF.
Cumulative risk of subsequent surgery after cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF).
Cumulative risk of adjacent-level subsequent surgery after cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF).
Between 7 and 10 years, 3 treatment-related AEs were reported in 3 CDA patients (subsidence—2; radiculopathy—1). The patient with radiculopathy underwent posterior fusion 9.5 years post-CDA surgery. One patient with subsidence received facet joint injections; the remaining CDA patient did not require an intervention. In the ACDF cohort, 4 AEs related to treatment were reported in 2 patients (kyphosis—1; decreased range of motion—1; diminished reflexes—1; muscle spasms—1), with none requiring an intervention. The cumulative risk of a treatment-related AE at 10 years was 18.6% in CDA vs 32.2% in ACDF (P = 0.024; Table 3).
Adjacent-Segment Pathology
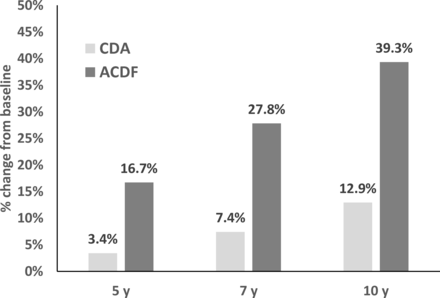
Similar to earlier periods, the progression to grade 3/4 RASP from baseline to 10 years was significantly lower in CDA vs ACDF (12.9% vs 39.3%, respectively; P = 0.006; Figure 7).
Percent of patients who progressed to grade 3/4 adjacent-segment pathology in cervical disc arthroplasty (CDA) vs anterior cervical discectomy and fusion (ACDF) (P = 0.006 at 10 y).
Clinical Outcomes
CDA and ACDF patients had similar preoperative NDI, pain, and SF-12 scores, and all patient-reported outcomes remained significantly improved from baseline for both treatments through 10 years (P < 0.05). At 10 years, patient-reported outcomes and change from baseline were generally better in CDA patients (Table 4). Specifically, the CDA group had greater improvement in SF-12 PCS than the ACDF group (15.7 vs 9.5; P = 0.004) at 10 years. Other differences between CDA and ACDF at 10 years were less than the minimal clinically important difference for NDI (15/100) and pain (10/100) and were not statistically significant. There was a trend toward higher NDI success in CDA, with rates of 85% vs 74.1% at 10 years (P = 0.25).
Patient-reported outcomes by treatment.
Patient satisfaction was high in both groups (CDA: 89.9% [78/79] vs ACDF: 85.2% [24/27]; P = 0.50). However, of those who were satisfied, a higher percentage of CDA patients reported they were “very satisfied” at 10 years (98.7% vs 88.9%; P = 0.05). Neurologic success was significantly higher in CDA (88%) vs ACDF (55.6%) at 10 years (P = 0.004), due primarily to diminished reflexes observed in ACDF patients at 5 years and beyond.
DISCUSSION
This postmarket study compares the safety and effectiveness of CDA with ACDF at 10 years. CDA continues to show superiority compared with ACDF for symptomatic cervical DDD at 10-year follow-up. The CDA group was statistically superior to ACDF in overall success rate (62.4% vs 22.2%), subsequent surgery (8.2% vs 33.3%), and neurologic success (87.8% vs 55.6%). At 10 years, CDA was noninferior to ACDF on all measures of clinical success.
Adjacent-Segment Pathology
The rates of secondary surgery at an adjacent level remained significantly lower in CDA patients up to 10 years after treatment. CDA had less progression of RASP from 7 to 10 years than in ACDF. In this study, grade 3/4 RASP occurred in 12.9% of CDA patients (1 and 2 levels combined) compared with 39.3% in ACDF.
One of the major concerns after ACDF is degeneration of the adjacent segments that can lead to reoperation to relieve associated symptoms.16 CDA has been shown to have lower rates of adjacent-segment degeneration13,17–19 and lower rates of subsequent surgery at adjacent levels13,20–26 compared with ACDF.
CDA can reduce the incidence of adjacent-segment pathology by preserving segmental motion and natural spinal kinematics. In vitro studies have shown that adjacent-segment motion, intradiscal pressure, and facet joint loading do not change after CDA.2,27 On the other hand, when motion in treated segments is eliminated by fusion, the adjacent discs may experience increased loads and stresses, resulting in hypermobility.4,6–8 These kinematic changes may initiate or accelerate degeneration in the untreated adjacent segments.28 The Mobi-C has shown maintenance of motion out to 10 years and minimal progression of RASP after 5 years.14
Whereas the progression of RASP is a radiographic finding that may not be directly associated with clinical outcomes, other studies have reported adjacent-level subsequent surgery as a proxy for clinically symptomatic adjacent-segment pathology. In this study, the rate of adjacent-level surgery at 10 years was 3.1% in CDA vs 20.5% in ACDF. The rate of adjacent-level surgery for ACDF in this study is consistent with the 2.4% to 2.7% per year reported in the literature.29,30 Long-term studies of CDA have reported adjacent-level surgery occurring in 4.5% to 13.8% of patients, compared with rates of 16% to 24% in ACDF controls.31–34 Hilibrand et al5 estimated that 25.6% of ACDF patients develop significant adjacent-level disease within 10 years of ACDF; remarkably similar to rates of adjacent-level surgery reported in the current study. Other studies have reported a cumulative incidence of adjacent-level surgery ranging from 21% to 37% at 10 years after ACDF.29,35–37 Recent RCTs have identified ACDF as the primary factor contributing to adjacent-segment pathology and adjacent-level surgeries compared with CDA.26,30,38
Several meta-analyses have confirmed that CDA has lower rates of clinical adjacent-segment pathology (CASP) leading to adjacent surgery. Chang et al25 included the data from RCTs for a robust review of 1864 patients treated with CDA and 1572 treated with ACDF. They reported combined CASP of 3.1% (range, 0.0%–7.1%) for CDA and 6.0% (range, 1.0%–11.9%) for ACDF.25 Luo et al showed CASP was 2.6% after CDA vs 6.0% in ACDF, with a significant difference in favor of CDA (P < 0.0001; OR = 0.43; 95% CI = 0.29, 0.64).18 In a separate meta-analysis, Xu et al39 showed CASP was 2.4% after CDA vs 4.5% in ACDF (OR = 0.52; 95% CI = 0.30, 0.87; P = 0.01). Most recently, Deng et al performed meta-analysis on 8 RCTs with follow-up of >48 months.40 Their analysis included 1334 patients with CDA and 1061 patients treated with ACDF. They reported overall CASP of 3.6% for CDA and 9.5% for ACDF (OR = 0.38; 95% CI = 0.27, 0.53; P < 0.0001).
Long-Term Safety and Effectiveness of CDA
Both CDA and ACDF demonstrated sustained improvement of NDI, pain scores, and SF-12 from preoperative to 10 years. CDA and ACDF patients had similar preoperative NDI, pain, and SF-12 scores, and all patient-reported outcomes remained significantly improved from baseline for both treatments through 10 years (P < 0.05). The percentage of patients who maintained their neurological function also remained stable.
At 10 years, patient-reported outcomes and change from baseline were generally better in CDA patients. Specifically, the CDA group had greater improvement in SF-12 PCS than the ACDF group (15.7 vs 9.5; P = 0.004) at 10 years. Other differences between CDA and ACDF at 10 years were less than the minimal clinically important difference for NDI (15/100) and pain (10/100) and were not statistically significant. These results suggest that CDA continues to be a clinically sound alternative to cervical fusion. The Mobi-C has been compared with ACDF for 1- and 2-level cervical disc disease out to 7 years after surgery in a multicenter, prospective, randomized IDE trial. Postoperative outcomes demonstrate statistically significant improvement in NDI, arm and neck pain, and SF-12 at 24 to 84 months in CDA compared with ACDF, especially after 2-level treatment.9–13
Limitations
The inclusion and exclusion criteria of the IDE trial may be considered a limitation of this study. For example, our study patients had no substantial pathology or degeneration adjacent to the treated levels, and patients with a prior fusion or other spinal surgery were not included. As with all IDE studies of CDA, the purpose of standardized inclusion criteria was to enroll patients without significant conditions that could confound the comparison with ACDF.
This study was based on a subset of the Mobi-C IDE trial; therefore, the patients and results from these sites may not represent the larger IDE trial. The randomization plan was applied independently at each center; therefore, treatment assignment at each center was independent and unbiased. This was verified by comparing the preoperative characteristics between CDA and ACDF, which showed a uniform distribution of patients between treatments at the 3 sites. We also compared baseline characteristics between the 3 centers and the remaining patients from the IDE trial who were not included in this subgroup to show that this subset was representative of the original FDA study cohort. The comparison of baseline characteristics between the 3 centers and the remaining patients from the IDE trial found no significant differences in preoperative characteristics between these patients and the original FDA cohort. Additionally, the 7-year outcomes in this cohort were consistent with outcomes reported for the entire IDE cohort at 7 years.
The attrition between 7 and 10 years could have affected the outcome of the study. Follow-up at 10 years was not obtained from 32 (23%) patients who were considered eligible for this postmarket study. The rate of attrition was higher in the ACDF control group. Eleven patients were alive and successfully contacted but declined to participate, although some who opted out may have done so because they were doing well and did not want to return for follow-up. However, the sensitivity analysis supported the results of the original analysis in over 93% and 81% of scenarios for study success and subsequent surgery, respectively. Those scenarios, where the study conclusions changed, represent extreme departures from data missing at random. Though plausible, these scenarios are very unlikely. With these considerations, the sensitivity analysis results support the validity of the treatment effect found in the original analysis.
This study was not designed to conclude that the results of our ACDF control group are representative of all ACDF techniques. Like many published RCTs of cervical arthroplasty, this study was undertaken to demonstrate noninferiority and, if appropriate, superiority of the Mobi-C disc when compared with ACDF. Although various ACDF techniques with a variety of graft options exist, studies designed to evaluate new cervical discs for FDA acceptance were required to use a single ACDF control that was an on-label application of current technology and generally accepted as the “gold standard” available at the time the study was designed and enrollment begun, in this case, 2004.
CONCLUSIONS
Ten years after surgery, CDA has significantly lower rates of subsequent surgery and adjacent-segment pathology. Our results through 10 years demonstrate that CDA continues to be a safe and effective surgical alternative to fusion. The significantly lower risk of subsequent surgery after CDA has the potential to greatly reduce the overall burden to the patient and health care system.
Acknowledgments
Data analysis and preparation of the draft manuscript, tables, and figures were performed by William B. Dolman, MS (ZimVie Spine).
Footnotes
Funding The device manufacturer, Zimmer Biomet, initiated and funded the Mobi-C© FDA IDE clinical trial and postapproval study. Zimmer Biomet contributed to the design and conduct of the study, and provided assistance with analysis of data, manuscript preparation, and review. ZimVie Spine paid the article processing charge. The authors received no additional financial support for the research and authorship of this article.
Declaration of Conflicting Interests Pierce Nunley reports royalties/licenses for Spineology, Stryker, ZimVie, Camber Spine and Accelus; consulting fees from Spineology, Camber Spine, Accelus, Centinel Spine, Intrinsic Therapeutics, Providence Medical, Neo Spine, NuVasive, NG Medical, ReGelTec, and Spinal Elements; payment/honoraria from Spineology, Camber Spine, Intrinsic Therapeutics, Providence Medical, and Neo Spine; leadership/fiduciary role for 3Spine; stock/stock options from Spineology, Camber Spine, ReGelTec, and 3Spine; and patent holder and consultant for Zimmer Biomet. Michael Hisey reports royalties/licenses; payment/honoraria and support for meetings/travel for lectures, presentations, speakers bureaus, manuscript writing, or education events; and patents for Zimmer Biomet. Micah Smith has nothing to disclose. Marcus Stone reports consulting fees from Spineology, Camber Spine, Organogenesis, Vyrsa, and ReGelTec; payment/honoraria and support for meetings/travel for lectures, presentations, speakers bureaus, manuscript writing, or education events from Spineology and Organogenesis; support for attending meetings/travel from Spineology; and a leadership or fiduciary role in Clinical Research I/O and the NASS Section on Surgical Engineering.
Ethics Approval Institutional review board approval and patient informed consent were obtained at each investigational site.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2023 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.