Abstract
Background Traditionally, spinal degeneration and injury have been associated with abnormal intervertebral motion; thus, treatment for lowback pain has centered on prevention of motion through spinal fusion. Although the rate of successful spinal fusions is improving, complications such as adjacent-level syndrome emphasize the need to develop alternatives for treating spinal degeneration. In an effort to improve the clinical outcomes associated with such treatment, we hypothesized that spinal stabilization and a consequent reduction in symptoms is achievable without the harsh restrictions to spinal motion imposed by fusion. This idea was based on the principle of the neutral zone and the neutral zone hypothesis of back pain.
Development Performance requirements for a novel device were determined through a series of biomechanical experiments. From these data, the Stabilimax NZ was developed to provide stabilization to a degenerated or surgically destabilized spine while maintaining the maximum possible total range of motion. Applied Spine Technologies Inc has tested 70 bilateral assemblies of the final design of the Stabilimax NZ, and all exceeded the biomechanical, static, fatigue, wear, and histological requirements necessary to initiate clinical investigation.
Discussion The Stabilimax NZ device has been systematically designed and tested under protocols developed by Applied Spine Technologies in conjunction with Panjabi, Patwardhan, and Goel. The device decreased the neutral zone in destabilized spines while maintaining substantial range of motion.
Clinical Relevance Development testing has been submitted to the US Food and Drug Administration and permission obtained to initiate an investigational device exemption trial to clinically investigate the efficacy of the Stabilimax NZ device.
INTRODUCTION
This commentary is a review of the authors’ hypothesis of back pain and the subsequent development of a dynamic stabilization device based on this hypothesis. Because the device is currently undergoing clinical investigation in an IDE trial, no conclusive results are available to report. A summary of the development efforts undertaken to date is provided here.
At some point in their lives, approximately 80% of adults will suffer acute back pain as a result of the normal degenerative process of the spine.1 As symptoms progress, lumbar surgery may become an option for treatment. In fact, doctors perform more than 200,000 spinal fusion operations each year in the United States to address severe physical disability associated with low-back pain. Two thirds of these procedures are performed in the lumbar spine.2, 3
BACKGROUND
Albee pioneered lumbar spinal fusion in 1911 to treat degenerative spinal disease.4 In 1933, Burns introduced the anterior lumbar interbody fusion technique.5 Later that decade, metallic implants were first utilized to provide supplemental fixation for fusion procedures. Recently, with the advent of bone morphogenetic proteins, successful fusion rates have approached 100%. Despite the progress over the past 70 years in the surgical technique for spinal fusion, the effectiveness of lumbar fusion to decrease pain and improve functioning has yielded mixed results when assessed through long-term follow-up of clinical outcomes.
In 1960, Harmon published 10-year follow-up results of lumbar disc excision and fusion in 30 patients.6 Harmon reported an osseous union (fusion) rate of 82.8% and an unsatisfactory clinical outcome in 3 of the 30 patients, a 90% success rate. More than 30 years later, in 1994, Mardjetko performed a metaanalysis of studies of lumbar fusion outcomes. He reported that posterior fusion without instrumentation produced fusion rates of 86% and a satisfactory clinical outcome of 90%.7 These findings are similar to those yielded by Yuan's meta-analysis of pedicle screw fusion outcomes. This study, also published in 1994, reported a 93% fusion rate and an 86% satisfaction rate when decompression and fusion included pedicle screw instrumentation.8 When compared with Harmon's surgical results, Mardjetko's and Yuan's recent studies highlight the fact that significant improvements in fusion rates have not translated to equivalent improvements in patient outcomes or satisfaction.
Long-term clinical outcomes of lumbar fusion have not reflected the significant progress made in technique and instrumentation. One explanation for the discrepancy lies in the effect that the surgical procedure has on spine biomechanics. When a vertebral joint is permanently fused, the vertebral levels adjacent to the fused joint are subjected to additional loads and stresses during routine activities.9 Adjacent-level syndrome occurs when the spinal joints adjacent to the site of the original fusion degenerate. It is a potential side effect of lumbar fusion that may result in the need for additional surgery. MacDougall's 2003 study of 178 patients with a minimum 10-year followup reported an 18% rate of adjacent segment degeneration, requiring subsequent surgery.10 In addition, only 70% of the patients surveyed judged their initial surgery to be a success 10 years later. Although the rate of successful fusions may be improving, complications such as adjacent level syndrome, as reported by MacDougall and others, emphasize the need to develop alternatives for treating spinal degeneration.11–13
HYPOTHESIS
In an effort to improve the clinical outcomes associated with such treatment, we hypothesized that spinal stabilization and a consequent reduction in symptoms is possible without the harsh restrictions to spinal motion imposed by fusion. This idea is based on the principle of the neutral zone and the neutral zone hypothesis of back pain.
THE NEUTRAL ZONE
One consequence of both tissue degeneration and surgical injury is abnormal intervertebral motion, which may be associated with pain in the lumbar spine. The change in spinal motion associated with spinal injury has been evaluated in a series of biomechanical studies. We defined the neutral zone as a region of intervertebral laxity around neutral posture.14 In a series of published articles, Panjabi evaluated the effects of injury, degeneration, and stabilization on the neutral zone of the spine.14–18 The neutral zone is very sensitive to changes in the spinal tissues and is therefore a clinically important measure of spinal instability. These evaluations, along with continual investigation into the interconnection of spinal biomechanics and clinical symptoms, has led to the neutral zone hypothesis of back pain.19–21 This hypothesis has, in turn, been used to provide guidance for the development of an alternative means of spinal stabilization.
This alternative method is based on the hypothesis that an increase in the neutral zone (laxity of the spine around neutral posture) because of injury or degeneration causes a cascade of effects that ultimately results in increased loading of various spinal components, accelerated degeneration, and back pain.22 To develop successful alternatives for treating spinal disorders, we investigated the interaction between the biomechanics of the spine and the mechanical parameters of the device.
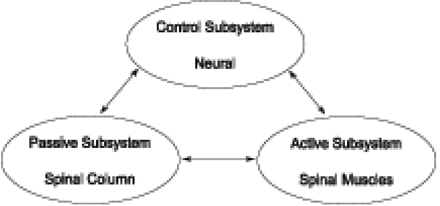
To understand the neutral zone principle, it is necessary to understand the 3 subsystems of spinal stability19: the passive subsystem (spinal column), the active subsystem (spinal muscles), and the control subsystem (neural) (Figure 1). Under normal conditions, these 3 subsystems maintain the mechanical stability of the spine. Damage or dysfunction in one subsystem requires the other 2 subsystems to compensate.
The 3 subsystems of the spinal stability system.19
Early studies focused on the effect of spinal injury on the stability of the spinal column (passive subsystem). The removal of a disc's nucleus produced an increase in flexion, lateral bending, and axial rotation.15 Other researchers, using similar models of spinal injury, have also observed that degeneration and trauma produced multidirectional laxity in the spinal column.23
The contribution of the spinal muscles (active subsystem) to the stability of the spine has also been investigated. After each injury, simulated muscle forces applied to the spinous process reduced the neutral zone to almost its intact value, without significantly affecting ROM.24 This finding has been supported by other research,25 and the results suggest that muscles in an injured spine perform extra work predominantly to restabilize the neutral zone, not to restrict the overall ROM.
The third spinal stabilizing subsystem (neuromuscular control) has been evaluated by comparing cohorts of patients with back pain to patients without back pain. Marras has demonstrated that there is a higher level of muscle activity in those patients experiencing back pain.26 It is theorized that this increase in muscle activity is triggered by the neural subsystem's responding to maintain the needed mechanical stability not being provided by the degenerated or injured passive subsystem. When muscles are recruited to compensate for the laxity of the spine, dysfunction and low-back pain appear to result.20
Traditionally, spinal degeneration and injury have been associated with abnormal intervertebral motion, which meant that the treatment for low-back pain was centered on the prevention of motion through spinal fusion. A review of the available literature reveals conflicting data about the relationship between an increase in spinal range of motion and the development of low-back pain.27–30
To examine the effect of a fixation system on the neutral zone and total ROM, we applied an external fixator to an isolated cervical spine preparation. The fixator decreased the neutral zone by 68.8%, substantially greater than the 39.3% decrease observed in ROM.18 This may indicate that when back injury occurs, the neutral zone is increased to a greater extent than the ROM; in a similar manner, the fixation of the spine decreases the neutral zone to a greater extent than it decreases ROM. Thus, change in the neutral zone may be a more significant indicator of spinal stability/instability than the ROM.20 This principle provided the framework for the development of a device that preferentially decreased the neutral zone while substantially maintaining physiological ROM.
DEVICE DEVELOPMENT
Pedicle screw-based dynamic stabilization systems are surgical devices that are implanted in the posterior spine. These devices work by preserving flexibility between the lumbar vertebrae while adding stability to the affected joints. The dynamic stabilization devices currently available have been developed in a variety of ways. The authors conceived a device that would be designed with mechanical properties derived through iterative experimental validation to have a specific predetermined effect on spine biomechanics. Thus far, it appears that this novel method of directly linking a hypothesis of back pain to the functionality of a stabilization device, and then designing it to experimentally determined criteria, is unique.
The design of this device was undertaken with keen interest in 3 areas of development:
Providing stabilization to a degenerative or surgically destabilized spine while maintaining the maximum possible range of spinal motion. Stabilization has been defined as reduction of the neutral zone to sub-intact levels and preservation of total motion at approximately 80% of intact levels.
Designing an associated instrument system compatible with minimally destructive surgical methods, such as the Wiltse technique, to maintain the innate stabilizing capacity of the muscles.31, 32
Verifying suitable device fatigue strength, wear resistance and bio-compatibility through validated testing protocols.
In conjunction with Panjabi, Applied Spine Technologies Inc. (New Haven, Conn) has developed a device, the Stabilimax NZ, in accordance with the neutral zone principle of spinal biomechanics. The device, a posterior pedicle screw-based dynamic stabilization system, features dual concentric springs combined with a ball-and-socket joint on each end. The device is designed to enhance the resistance of the passive spinal system around neutral posture (the neutral zone) while maintaining the maximum possible range of motion.
EXPERIMENTAL VERIFICATION
Panjabi conducted initial development experimentation to determine optimal device parameters for spring stiffness and associated interpedicular travel. Patwardhan, Goel, and Panjabi conducted subsequent studies to verify the effect of the device following progressive destabilization procedures. Applied Spine Technologies Inc and several contract facilities performed static, fatigue, wear, and in vivo histologic evaluation to demonstrate that the device met strength, durability, and compatibility requirements.
The characteristics of the device were optimized through a set of biomechanical studies. Optimal spring stiffness for the device was determined in both the intact spine and the spine destabilized by nucleotomy and laminectomy with partial facetectomy. Optimal interpedicular travel of the device was established for both compression of the device during spinal extension and elongation of the device during spinal fiexion. Optimal spring stiffness of 90 N/mm and associated travel were determined when the device was capable of consistently reducing the neutral zone to the pre-destabilized levels regardless of specimen condition while maintaining the maximum possible range of spinal motion.
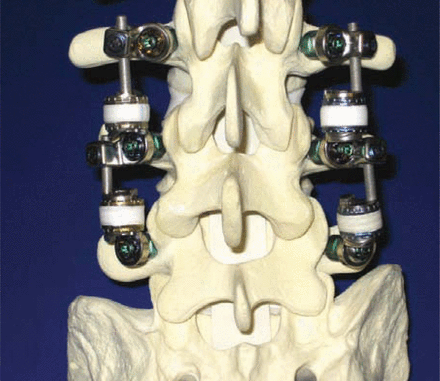
Patwardhan tested 6 human cadaveric L2-L5 specimens to determine the effect of the Stabilimax NZ (Figure 2) on range of motion, neutral zone, and axis of rotation of the spine. The flexibility protocol with both a 0 N and 400 N follower load was applied for flexion/extension, lateral bending, and axial rotation, as previously described by Patwardhan et al.33 Specimens were tested (1) intact, (2) after destabilization consisting of L3 laminectomy, bilateral L3-L4 foraminotomy, and L3-L4 nucleotomy, and (3) after implantation of Stabilimax NZ at L3-L4. Destabilization showed an increase in ROM and an increase in the neutral zone. With the application of Stabilimax NZ, the range of motion and neutral zone of the instrumented level were reduced, stabilizing the motion of the spine. The axis of rotation, measured from fluoroscopic images taken at maximum rotations as previously described by Patwardhan,34 demonstrated a 1.9-mm posterior shift from comparable intact axis of rotation with a standard deviation of 1.6 mm. It is important to note that more motion was maintained after instrumentation with the Stabilimax NZ than has been found with fusion constructs.35, 36 (A more detailed analysis of this data has been accepted for presentation at the 2007 Annual Meeting of the International Society for the Study of the Lumbar Spine.)
Two-level Stabilimax NZ.
Goel performed a verification study using his previously validated finite element model of the ligamentous lumbar spine, levels L3-S1, instrumented with the Stabilimax NZ. The validity of the finite element model analysis was supported by a strong correlation between the motions predicted by the finite element spinal model and the in vitro cadaver experiments previously conducted by Patwardhan. We used this model to evaluate the complex loading on internal elements of the device and spinal tissues otherwise impossible to quantify. The finite element model analysis demonstrated that the Stabilimax NZ device provided approximately twice the motion compared with a typical fusion system in response to moments placed on the superior vertebra. Also, with preload, maximum Von Mises stress observed in the pedicle screw was 121 MPa with the rigid fusion system, compared to 36 MPa with the Stabilimax NZ. (A more detailed analysis of this data has been accepted for presentation at the 2007 Spine Arthroplasty Society Annual Meeting.)
Panjabi performed cadaveric testing on 6 human lumbar spine sections, T12–S1, under the flexibility and hybrid testing protocols. This testing provided a direct comparison to a fusion construct at the implanted level and also evaluated the comparative adjacent level effect in the spine specimens with fusion and with Stabilimax NZ. Following intact testing, the spine was destabilized by a total laminectomy at L4 plus a bilateral partial facetectomy at L4-L5. After implementation of the Stabilimax NZ at the L4-L5 level, the motion of the spine was stabilized in flexion/extension and lateral bending. The average adjacent-level effect in the form of increased motion for Stabilimax NZ was approximately half that for fusion (unpublished data).
Performance requirements for the Stabilimax NZ have been based on the long clinical history of pedicle screw fusion systems and biomechanical data. We tested 70 bilateral assemblies of the final design to demonstrate the safety of the device, and all exceeded static, fatigue, wear resistance, including particle characterization, and histological requirements. We have submitted all development testing to the US Food and Drug Administration and obtained permission to initiate an IDE trial to clinically investigate the efficacy of the Stabilimax NZ device.
In an effort to improve on the long-term clinical outcomes yielded by current fusion technology, Panjabi has developed a dynamic spine stabilization system based on the neutral zone hypothesis of back pain. Under protocols developed by Applied Spine Technologies in conjunction with Panjabi, Patwardhan, and Goel, the Stabilimax NZ device has been systematically developed and tested. The device decreased the neutral zone in destabilized spines while maintaining substantial range of motion. Additionally, we have evaluated the device rigorously to demonstrate its strength, durability, and biocompatibility required for clinical use. The Stabilimax NZ provides a new alternative for the treatment of spinal degeneration, one that invites further investigation.
Footnotes
Institutional review board approval was not required.
- Received January 3, 2007.
- Accepted February 26, 2007.
- Copyright SAS - Spine Arthroplasty Society 2007
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License, permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Manohar M. Panjabi is cofounder and a paid consultant of Applied Spine Technologies Inc, New Haven, Conn.
Jens Peter Timm is an employee of Applied Spine Technologies Inc.