Abstract
Background Anterior cervical discectomy and fusion (ACDF) is known to elicit adverse biomechanical effects on immediately adjacent segments; however, its impact on the kinematics of the remaining nonadjacent cervical levels has not been understood. This study aimed to explore the biomechanical impact of ACDF on kinematics beyond the immediate fusion site. We hypothesized that compensatory motion following single-level ACDF is not predictably distributed to adjacent segments due to compensation from noncontiguous levels.
Methods Six fresh-frozen cervical spines (C2–T1) underwent fluoroscopic screening and sagittal and coronal reformats from computed tomography scans and were utilized to grade segmental degeneration. Each specimen was tested to 30° of flexion and extension intact and following single-level ACDF at the C5–C6 level. The motions of each vertebral body were tracked using 3-dimensional (3D) motion capture into an inverse kinematics model, facilitating correlations between the 3D reconstruction from computed tomography images and the 3D motion capture data. This model was used to calculate each level’s flexion/extension range of motion (ROM).
Results Single-level fusion at the C5–C6 level across all specimens resulted in a significant motion reduction of −6.8° (P = 0.002). No significant change in ROM occurred in the immediate adjacent segments C4–C5 (P = 0.07) or C6–C7 (P = 0.15). Hypermobility was observed in 2 specimens (33%) exclusively in adjacent segments. In contrast, the other 4 spines (66%) displayed hypermobility at noncontiguous segments. Hypermobility occurred in 42% (5/12) of the adjacent segments, 28% (5/18) of the noncontiguous segments, and 50% (3/6) of the cervicothoracic segments.
Conclusion Single-level ACDF impacts ROM beyond adjacent segments, extending to noncontiguous levels. Compensatory motion, not limited to adjacent levels, may be influenced by degenerative changes in noncontiguous segments. Surprisingly, hypermobility may not occur in adjacent segments after ACDF.
Clinical Relevance Overall, the multifaceted biomechanical effects of ACDF underscore the need for a comprehensive understanding of cervical spine dynamics beyond immediate adjacency, and it needs to be taken into consideration when planning single-level ACDF.
Level of Evidence 4.
Introduction
Anterior cervical discectomy and fusion (ACDF) has been established as a gold standard in surgical interventions for cervical radiculopathy or myelopathy, particularly for patients unresponsive to nonsurgical treatments.1,2 ACDF has shown to be efficacious in the short-term treatment of these patients, but long-term outcomes can face challenges due to breakdown and degeneration at motion segments adjacent to the fused level. This phenomenon, called adjacent segment degeneration (ASD), significantly impacts the patient’s musculoskeletal health and quality of life and imposes substantial costs on the health care system.3 ASD is a common complication after ACDF, presenting with an overall incidence of 14.5% ranging from 6.2% to 47.1%, as reported in a systematic review of 3136 patients by Broida et al.4
ASD can be categorized into 2 types. The first involves radiographic degenerative changes in the adjacent motion segment, which are not necessarily correlated with observable clinical symptoms. The second type encompasses clinical sequelae, such as the development of new radiculopathy or myelopathy attributed to radiographic alterations at the level adjacent to the prior anterior arthrodesis.3 Lawrence et al estimated the annual incidence of symptomatic degeneration in the cervical spine after ACDF to be between 1.6% and 4.2%.5 Wu et al conducted an extensive study revealing a consistent trend of recurrent ACDF surgeries for ASD, resulting in an annual incidence of approximately 0.8%—notably lower than the reported incidence of symptomatic ASD.6
The relationship between ASD and aberrant cervical alignment has been a focal point for research, with several risk factors hypothesized as contributing to ASD development, including age, sagittal alignment, cervical spine range of motion (ROM), and spinal canal stenosis.7 Existing data suggest potential causative factors, such as increased intradiscal pressure and hypermobility at segments above and below a fusion.8,9
However, controversy persists regarding the etiology of ASD, with debates centering on whether it stems from untoward biomechanical effects postfusion or is more related to the natural progression of cervical spondylosis over time.5–7 It is critical to understand that after a single-level ACDF, 5 unfused motion segments remain in the subaxial cervical spine (C2–T1) between the cranial occipitocervical complex (occiput to C2) and the more rigid thoracic spine caudally. Therefore, the potential exists for biomechanical compensation due to the fused segment anywhere along this region leading to numerous investigations on the kinematics of the cervical spine after fusion. It was shown in human patients that C5–C6 arthrodesis does not affect the total ROM in adjacent vertebral segments, but it does alter the distribution of adjacent-segment motion toward more extension and less flexion superior to the arthrodesis and more posterior translation superior and inferior to the arthrodesis during in vivo functional loading.10 Notably, data suggest an increase in motion not only at the segments immediately cranial and caudal to an ACDF but also at levels not immediately adjacent to the fusion.11,12 However, biomechanical investigations often overlook a comprehensive analysis of all levels.13,14
Furthermore, biomechanical investigations focusing on the kinematics of adjacent segments following spinal fusion using cadaveric models have encountered challenges due to inadequately justified methodologies regarding pertinent input parameters.15
Our study aimed to examine the impact of single-level ACDF on the entire subaxial cervical spine. To accomplish this, we employed 3-dimensional (3D) motion capture techniques coupled with anatomical reconstructions based on computed tomography (CT). Our hypothesis was that compensatory motion following single-level ACDF would extend throughout the cervical spine, even in noncontiguous motion segments.
Materials and Methods
A custom apparatus for loading cadaveric spines while tracking the overall motion of the spine and the 3D motions of the individual vertebrae was developed based on an earlier version.16,17 The testing approach allowed for simultaneous measurements of the necessary motions required to define the kinematics and alignment of the cervical spine and how both were altered by fusion. Fluoroscopic screening of cadaveric cervical spine specimens focused on identifying specific signs in the x-ray images to detect conditions that made them inappropriate for testing. Specimens were excluded if they showed evidence of trauma, such as fractures or dislocations; scoliosis, identified by an abnormal lateral curvature of the spine; tumors, seen as irregular masses or bone destruction; deformities, which could include unusual spinal shapes or alignments; or advanced spondylosis, indicated by significant joint degeneration or bone spurs.
Six fresh-frozen cervical spines (C2–T1) were selected, thawed, and dissected, leaving the disco ligamentous anatomy intact. The superior aspect of C2 and the inferior aspect of T1 were potted in epoxy (Bondo, 3M, Maplewood, MN). Clusters of retroreflective markers were rigidly fixed to each vertebral body. A previously reported reliable scoring system18 was utilized to assess degeneration in the 6 specimens’ intervertebral discs and facet joints from C2 to T1 using sagittal and coronal reformatted CT images.
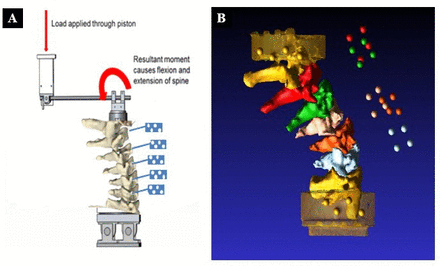
To examine segmental motion, each specimen was placed in a custom fixture on a servohydraulic load frame (MTS, Eden Prarie, MN; Figure 1a and Figure 2). The set-up consisted of a hydraulic actuator mounted on the crosshead connected to an upper mounting fixture assembly with a linear bearing and pinned assembly connected to a shaft attached to the spine. The linear bearing provided a frictionless connection to the shaft to reduce applied shear forces during the movement of the actuator. The load axis of the actuator was eccentric to the spine, allowing either a compressive flexion or extension bending moment to be applied. A rotational displacement transducer was attached to the upper pinned assembly to measure the global rotation of the spine.
(A) Schema of the test set-up showing the cervical spine specimen and motion capture markers (in blue). (B) Computed tomography reconstruction of a spine showing vertebrae.
Photographs of the testing apparatus with servo-hydraulic loading frame demonstrating hydraulic actuator mounted on crosshead connected to the mounting fixture in a lateral (A) and anterior (B) view.
Each specimen was tested intact from 0° to 30° of flexion and 0° to 30° of extension as measured by the rotational displacement transducer, each test consisting of 5 complete cycles of a sinusoidal loading curve at a frequency of 0.1 Hz (6°/s). A loading rate slower than physiological was selected for the frequency to ensure comprehensive data collection, increase the number of data points per degree of rotation, and minimize potential damage to the specimens.
The first 4 cycles were designated as conditioning cycles, facilitating the stabilizing of each spine’s viscoelastic properties. The motions of each vertebral body were tracked from the retroreflective markers using a 3D motion capture system (Motion Analysis Corp, Santa Rosa, CA). Data were reported from the fifth sinusoidal cycle. Following testing of the intact specimen, a complete discectomy at C5–C6 was performed, and a titanium interbody cage (Titan Spine, Mequon, WI) was placed into the disc space with anterior cervical locking plate fixation (DePuy Synthes, West Chester, PA) applied to simulate a fusion at that level.
Data Analysis
Each spine underwent CT with the marker clusters in place before and after all testing. The pretest CT image was used to evaluate the intact kinematics, and the posttest CT image was used to evaluate the fused kinematics. This allowed us to compensate for markers being inadvertently bumped out of position during the fusion procedure. These data provided the inputs to an inverse kinematics model that was used to correlate the 3D reconstruction of the CT images to the 3D motion capture data (Figure 1b). Although kinematics could have been calculated using the motion capture data directly, modeling each test helped us to visualize and verify the motions of each specimen. The model’s outputs were the flexion and extension of each segment and were measured relative to the neighboring level. For every spinal level, the rotation around the mediolateral axis concerning the neighboring segment was assessed at the maximum points of flexion and extension, both in the intact and fused spine scenarios. The ROM for each motion segment and the overall ROM of the entire spine were calculated. Alterations in intervertebral ranges of motion were then compared before and after the surgical intervention to simulate the single-level ACDF. A decrease in ROM >2° was classified as hypomobility; an increase of ≥2° was classified as hypermobility.18
Statistical Analysis
A generalized linear model (GLM) was used to analyze the change in pre- and postkinematics measurements. The GLM approach allows for more flexibility in analyzing a wide array of data types. Additionally, the GLM also accounts for the clustered nature of the data. In this analysis, the adjustments were made to account for the levels within each of the cervical spines of each specimen used. Analyses were performed using SPSS version 23.0 (IBM Corporation, Armonk, NY). Statistical significance was set to a critical alpha level of 0.05.
Results
Thirty nonfused cervical segments were examined across 6 fresh-frozen cervical spines (C2–T1), which were either adjacent or noncontiguous after fusion at the C5-C6 level.
Changes in the Fused Level
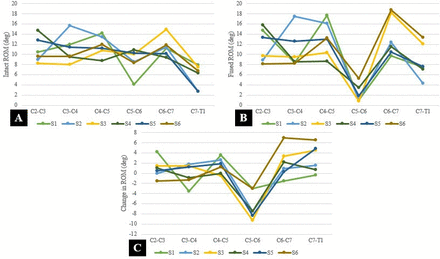
Effective reduction in motion was observed at the C5–C6 level across all specimens after ACDF, with a significant (P = 0.002) reduction in the total arc of motion of 6.8° at the fused level (C5–C6) representing an average reduction of motion of 73% (range 36%–92%; Figure 3).
(A) Preoperative range of motion (ROM) of each segment per specimen. (B) Postoperative ROM after fusion of C5-C6 of each segment per specimen. (C) Characteristic changes in motion in all 6 specimens after C5-C6 anterior cervical discectomy and fusion at each motion segment.
Changes at Adjacent and Noncontinuous Levels to the Fused Level
The 6 specimens displayed unique patterns of compensatory motion in the nonfused motion segments (Figure 3). A significant increase (P = 0.04) in motion was noted at the C7 to T1 motion segment in 50% (3/6) of the specimens (Table 1). Hypermobility was observed in 1 adjacent segment in all specimens, except for specimen 5 (Table 2). It was observed at only 1 level in 2 specimens, where specimen 2 exhibited increased motion only at the cranial adjacent segment (C4–C5), while specimen 4 exhibited hypermobility only at the caudal adjacent segment (C6 to C7).
Absolute average change in motion per segment after cervical fusion of C5–C6.
Segmental total motion for each specimen (C2–T1) in the intact state and fused state after anterior cervical discectomy with cage placement and plate fixation at C5–C6.a
In the remaining 4 specimens, hypermobility extended beyond the adjacent segments to noncontinuous ones. Specimens 3 and 6 exhibited heightened hypermobility in both caudal segments (C6–C7 and C7–T1), and specimen 5 showed hypermobility exclusively at C7 to T1, while motion in the adjacent segments remained unchanged.
Degenerative Findings
Degenerative alterations in the intervertebral discs and facet joints found in CT images during the experiment revealed degenerative changes that were not detected during the fluoroscopic screening of the cadaveric specimens.
A mild degree of disc degeneration was observed in 27% (8/30) of the segments, while moderate degeneration was present in 10% (3/30), with no instances of severe degeneration noted in any of the motion segments (Table 3).
Cumulative scores of radiographic grading (Rydman et al) of the extent of degeneration of cervical spine cadaveric specimens at each motion segment from C2 to T1.
As for facet degeneration, mild findings were identified in 20% (6/30) of cases, moderate degeneration was observed in 7% (2/30), and severe degeneration was evident in 10% (3/30) of the segments.
Relation of Degenerative Findings and Change in Motion
In specimen 1, moderate disc degeneration was evident on the CT image at the caudal adjacent segment (C6–C7), with hypermobility observed only at the segments cranial to the fusion.
Two specimens (3 and 5) revealed signs of severe facet arthropathy not evident in the pretesting fluoroscopic screenings. In specimen 5, without radiographic evidence of facet arthropathy, C7 to T1 was the only segment demonstrating hypermobility following fusion.
Discussion
Our investigation aimed to explore the biomechanical implications of single-level ACDF across the entire subaxial cervical spine (C2–T1), not limited to only the adjacent segments to the fusion. Our results strongly indicate that the effects of a single-level ACDF extend beyond the adjacent segments.
Our findings revealed hypermobility in 42% (5/12) of the adjacent segments and 28% (5/18) of the noncontiguous segments. This aligns with observations in another C5 to C6 fusion model, where motion distribution was “fairly equal” among segments, deviating from a disproportionate increase in immediately adjacent segments.11,12 In specimen 5 in our study, hypermobility was observed in the C7–T1 segment without signs of degeneration. In contrast, adjacent segments in other specimens, with stiffness and degenerative changes, influenced compensatory motion differently (cranially in specimen 1 and caudally in specimen 3). ACDF in specimen 1 reduced motion at C3–C4, indicating broader kinematic alterations. Notably, degenerative findings on CT did not impact compensatory motion in specimens 2, 4, and 6. This suggests that other factors affect distinct motion patterns, emphasizing the complexity of ACDF’s biomechanical impact.
The extent of degeneration, as evident in sagittal, coronal, and axial CT images of intervertebral discs and facet joints, likely contributed to variations in compensatory motion patterns across specific specimens. Theoretically, a motion segment undergoing degenerative changes, resulting in increased stiffness adjacent to an ACDF, could cause a redistribution of motion to noncontiguous segments.19 This premise is supported by the observed motions in specimen 5, suggesting a plausible link between degeneration and the redistribution of motion following ACDF.
Notably, our findings highlight that the average change in motion at the nonfused segments lacked a significant pattern. This underscores the complexity of the biomechanical response to single-level ACDF, with specific segments exhibiting unique behaviors. Further elucidating these nuanced patterns could contribute to a deeper understanding of the dynamic interplay within the cervical spine after ACDF. Previous cadaveric studies faced limitations by not encompassing the entire cervical spine (C2–T1), thus compensatory motion for the fused segment by the noncontiguous levels was overlooked.13,14,20–22
The origins and mechanisms of ASD have long been debated.8 One hypothesis suggests that spinal fusion alters adjacent segment biomechanics, supported by increased anteroposterior shear displacements observed in post-ACDF radiographs.23 Such hypermobility and shear forces could accelerate degeneration.24 Contrary to earlier beliefs, recent clinical data challenge the notion that ASD is confined to immediately adjacent segments. Song et al found no significant difference in symptomatic degeneration after ACDF between adjacent and noncontiguous segments, reshaping our understanding of ASD patterns.7 The clinical variability in ASD occurrence remains poorly understood, with genetic susceptibility as a possible explanation.25,26
Supporting the theory that altered biomechanics following fusion cause ASD, an entire industry of spinal arthroplasty devices and techniques have been developed for the cervical and lumbar spine in an effort to better preserve physiological biomechanics.23 Previous biomechanical studies supported this approach. For example, an increase in both intradiscal pressure and segmental ROM at the level adjacent to spinal fusion was found in cadaveric ACDF models.13,20,27 This heightened pressure is thought to impede the diffusion of essential nutrients from the endplate into the disc, potentially accelerating the degenerative process.28 However, associated clinical data have not been found, as evidenced by a recent randomized controlled trial that found no significant difference in the incidence of clinical ASD between cervical total disc replacement and ACDF at 5-year follow-up. This finding suggests that factors other than fusion itself may play a role.29
Potential limitations of this study include measurements that fell within the error margin of the biomechanical system.30 To minimize this limitation, the motion analysis measurement system of cameras was calibrated daily, resulting in a measurement accuracy of 0.15 mm in translation and 1° in rotation. Although the motion capture measurement errors were small, they can be increased after transforming the geometries from 1 measurement module (CT) to the motion capture system. The accuracy of angular measurements can be affected by the placement of motion markers, which were placed away from the end plates of the vertebral body. This can cause small errors to magnify over long distances, making it impossible to interpret calculated differences below a measurement accuracy of 2°. Therefore, the statistical significance of previous models based on only a few degrees of change in adjacent segment motion is questionable and likely falls within the measurement error of the testing apparatus.7
The reliance on fluoroscopic screening for cadaveric specimens is a potential limitation as it led to the oversight of degenerative facet and disc findings in 37% of cases, revealed only through subsequent CT images.27 This suggests an underestimation of pathological conditions, highlighting the need for caution when solely using fluoroscopy. Additionally, the absence of pretesting CT images could have contributed to sample heterogeneity. With an initial marker displacement rate of 100%, the implementation of CT scans after mechanical testing became imperative to ensure accurate marker placement. The omission of CT imaging in prior ACDF biomechanical studies raises concerns about potential errors due to marker displacement, emphasizing the significance of employing confirmatory imaging methods such as magnetic resonance imaging in future investigations for a comprehensive understanding of specimen degeneration and compensatory motion.11,20
To understand the enduring biomechanical repercussions of ACDF across the remaining nonfused portions of the cervical spine, meticulous consideration of all motion segments is indispensable. Many previous cadaveric studies did not include analysis of the entire subaxial cervical.9,13,14,30 However, any cadaveric study has inherent statistical and financial limitations that must be acknowledged as contributing to the observed data dispersion. Due to logistical constraints, the requisite number of cadaveric specimens to adequately represent the diverse ACDF population poses a formidable challenge. Furthermore, while cadaveric studies provide valuable insights, the biomechanical composition of a cadaveric spine may not faithfully replicate an in vivo motion segment. Factors such as the dynamic influence of muscles, metabolically active cells, and the varied viscoelastic properties of cellular and extracellular environments introduce additional intricacies that could cause deviations from the dynamic biomechanics of a living patient’s cervical spine. This underscores the necessity for circumspect extrapolation of study outcomes to living populations.
The present study utilized displacement control for testing for an intuitive reason—individuals will move their necks in an attempt to perform any given activity to a particular position rather than to achieve a particular load. However, the total 60° arc of motion in flexion and extension could have exceeded that motion at particular motion segments and forced them into the inelastic zone. The single plane of motion in flexion and extension was chosen to compare the data to the majority of the published biomechanical data on ASD following ACDF. Future models, including lateral bending and axial rotation, representing a more natural ROM, will provide additional insight into the compensatory motions of the cervical motion segments following ACDF.31
Conclusion
The impact of single-level ACDF extends beyond adjacent segments, involving noncontiguous levels. Compensatory motion after ACDF not being confined to adjacent levels leads to the possibility of noncontiguous levels being influenced by degenerative changes. Contrary to expectations, adjacent segments may not necessarily display hypermobility after ACDF. The presence of degenerative changes in the segmental intervertebral discs and facet joints plays a role in determining which segments compensate for motion loss after ACDF. In essence, the altered biomechanical effects of ACDF are multifaceted, emphasizing the need for a comprehensive understanding of cervical spine dynamics beyond immediate adjacency.
Acknowledgments
A grant from the Louis V. Gerstner Jr. Foundation funded this study.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
Disclosures F.C.S.A., H.J., J.N., T.J.C.P., P.F.O.L., and K.N.M. have no disclosures. D.R.L. reports royalties from NuVasive, Inc. and Stryker; private investments from HS2, LLC, Woven Orthopedic Technologies, Vestia Ventures MiRus Investment LLC, ISPH, LLC; consulting fees from Depuy Synthes, Vizeon, Inc.; scientific advisory board from Remedy Logic; and research support from Medtronic. T.M.W. reports royalties from Exactech, Inc. and Lima Corporate; is a Data Committee member of American Joint Replacement Registry; is a Board or Committee Member of OREF; and receives research support from Lima Corporate.
Ethics Statement The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written consent was obtained for use of deidentified images in publication.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2024 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.