Abstract
Background Lumbar spinal fusion surgeries are increasing steadily due to an aging and ever-growing population. Patients undergoing lumbar spinal fusion surgery may present with risk factors that contribute to complications, pseudarthrosis, prolonged pain, and reduced quality of life. Pulsed electromagnetic field (PEMF) stimulation represents an adjunct noninvasive treatment intervention that has been shown to improve successful fusion and patient outcomes following spinal surgery.
Methods A prospective, multicenter study investigated PEMF as an adjunct therapy to lumbar spinal fusion procedures in patients at risk for pseudarthrosis. Patients with at least 1 of the following risk factors were enrolled: prior failed fusion, multilevel fusion, nicotine use, osteoporosis, or diabetes. Fusion status was determined by radiographic imaging, and patient-reported outcomes were also evaluated.
Results A total of 142 patients were included in the analysis. Fusion status was assessed at 12 months follow-up where 88.0% (n = 125/142) of patients demonstrated successful fusion. Fusion success for patients with 1, 2+, or 3+ risk factors was 88.5%, 87.5%, and 82.3%, respectively. Significant improvements in patient-reported outcomes using the Short Form 36, EuroQol 5 Dimension (EQ-5D) survey, Oswestry Disability Index, and visual analog scale for back and leg pain were also observed compared with baseline scores (P < 0.001). A favorable safety profile was observed. PEMF treatment showed a positive benefit-risk profile throughout the 6-month required use period.
Conclusions The addition of PEMF as an adjunct treatment in patients undergoing lumbar spinal surgery provided a high rate of successful fusion with significant improvements in pain, function, and quality of life, despite having risk factors for pseudarthrosis.
Clinical Relevance PEMF represents a useful tool for adjunct treatment in patients who have undergone lumbar spinal surgery. Treatment with PEMF may result in improved fusion and patient-reported outcomes, regardless of risk factors.
Trial Registration NCT03176303
- pulsed electromagnetic fields
- lumbar spinal fusion
- PEMF
- adjunctive therapy
- bone stimulation
- pseudarthrosis
- failed fusion
Introduction
Electromagnetic radiation refers to the flow of electrons and the coupled magnetic field generated by the electron movement. Electrons move in differing frequencies or may present in a static state or flow, which influences the magnetic field. Manipulation of electromagnetic parameters impacts cellular excitability and physiology.1 Pulsed electromagnetic field (PEMF) stimulation represents a noninvasive, postoperative, interventional therapy that stimulates and accelerates healing capabilities in biological tissues.2–5 Direct activation of targets at the cellular level is recognized for its effects leading to improvements in clinical outcomes in a variety of medical indications. An abundance of literature exists on PEMF therapy and suggests an efficacious and safe treatment modality that may be used following postoperative spinal surgery to promote fusion and improve patient outcomes.6–9 Evidence from randomized controlled trials in multiple indications, including musculoskeletal injury, fibromyalgia, back pain, and osteoporosis, supports the effectiveness and safety of this technology.9–13
At the molecular level, it is postulated that PEMF works by electron transport stimulation across the cell membrane of osteoblasts, inducing an intracellular response that activates a myriad of signaling pathways. The benefits of PEMF therapy are ascribed to changes in inflammatory, regenerative, and protective cellular processes.14–17 PEMF engages a multitude of cellular signaling systems and impacts the biosynthesis of structural and signaling extracellular matrix components important in bone integrity and development.18 Direct effects on the structural integrity of bone and cartilage extracellular matrix enhance regenerative and repair mechanisms and reduce inflammatory insult.16,19–24 The postoperative effects on bone tissue proliferation and vascular flow are very appealing for spinal surgery initiatives.1 Collectively, these effects are thought to contribute to the clinical benefits observed across indications.
Spinal fusion is a prevalent surgical procedure for the treatment of spinal disease, injury, and pain. The goal of spinal fusion surgery is to immobilize the motion segment by bone graft transplantation, ultimately leading to bone restructuring and remodeling. Spinal fusion exploits natural tissue responses to accelerate proper bony fusion. These processes include inflammation, vascularization, osteoinduction, osteoconduction, and remodeling.25 Pseudarthrosis, the nonunion or failure of union after fusion surgery, is reported in up to 35% of patients and a leading complication.26,27 Nonsuccessful union results in loss of correction and instrumentation failure, increased postoperative complications, and reductions in patients’ quality of life.28 Many risk factors contribute to complications and pseudarthrosis following spinal surgery (eg, smoking, diabetes, osteoporosis, and advanced age). Efforts toward improving patient success may include administering PEMF postoperatively to improve fusion, which may result in reduced pain and improved function. PEMF therapy is noninvasive, is cost-effective, and has a favorable safety profile, which enables use outside of a clinical setting (ie, can be used in the patient’s home during recovery).
Clinical benefit has been reported in areas of musculoskeletal injury and disease, including multiple sclerosis, congenital pseudarthrosis, osteoporosis, osteoarthritis, bone fracture healing, cervical disc herniation, chronic pain, nerve regeneration, and fibromyalgia.29–35 Mooney et al evaluated the effect of PEMF in interbody lumbar fusion using an early model of the SpinalStim device in a randomized controlled trial design and found a 92% success rate in treated patients compared with 65% in control patients.7 The underlying mechanism of action coupled with published evidence supporting PEMF in musculoskeletal indications provides the rationale for the current study, which aimed to further investigate the effect of PEMF as an adjunct intervention postoperative to lumbar spinal surgery in patients with risk factors for pseudarthrosis.
Methods
Participants
Patients undergoing lumbar fusion surgery with 1 or more risk factors for nonunion were eligible for enrollment into the study (NCT03176303). Risk factors included multilevel (2 or more) fusion procedures, prior failed lumbar spine fusion, diabetes, osteoporosis, or current nicotine usage. Included patients were at least 18 years old and had a body mass index (BMI) ≤45 kg/m² at the time of consent. Patients were excluded from enrollment if they had a diagnosis of scoliosis (>30°); were currently undergoing treatment for malignancy or treatment in the past 5 years; displayed current alcoholism or drug abuse and/or any known current addiction to pain medications or medical marijuana; had any clinically significant finding that, in the opinion of the investigator, placed the patient at health risk, impacted the study, or affected completion of the study; had any psychiatric illness that prevented the patient from completing the assessments accurately; or were a prisoner.
Patients were enrolled across 10 centers throughout the United States. Patients who met the inclusion criteria, had none of the exclusion criteria, and provided their written informed consent were enrolled and treated with the SpinalStim device (Orthofix US LLC, Lewisville, TX, USA). Patients received PEMF therapy via SpinalStim, designed specifically for patient use in the home setting, for 6 months following surgery. SpinalStim is the only bone growth stimulation therapy device FDA-approved as both a lumbar spinal fusion adjunct and as a nonsurgical treatment for spinal pseudarthrosis. The SpinalStim device generates repeating burst groups of multiple pulses of electromagnetic energy by delivering time-varying electrical signals to a treatment coil, where the groups of multiple pulses repeat at a constant interval while a treatment session is active. SpinalStim provides 360° of PEMF treatment around the fusion site that evenly penetrates across tissue, bone, and fixation.36 No clinician oversight is necessary for use of the device. Patients initiated PEMF treatment within 14 days of surgery. This study was conducted in compliance with the protocol, Good Clinical Practice guidelines, and all other applicable regulatory requirements. This study was performed in adherence to the guidelines of the Declaration of Helsinki.
Study Design and Evaluated Parameters
This postmarket, prospective, multicenter (10 sites) study evaluated the safety and effectiveness of the SpinalStim device as an adjunct treatment to lumbar arthrodesis. Patients were required to use the PEMF device for 2 h/d for 6 months. The primary outcome measure was fusion status at the 12-month follow-up period as indicated by radiography and computed tomography (CT). Fusion status was determined by the site investigator using anterior/posterior (A/P), lateral, and flexion/extension radiographs in addition to CT (without contrast). Use of x-ray/CT imaging and clinical impression to evaluate fusion was done per each investigator’s standard of care. All treated levels had to demonstrate bony fusion for the patient to be considered a fusion success. Patient-reported outcomes including the Oswestry Disability Index (ODI), EuroQol 5 Dimension (EQ-5D) quality of life (QoL) survey, short form (SF-36) QoL survey, and visual analog scale for pain (VAS for back pain and leg pain) were collected as secondary outcome measures. Adverse events were collected for safety assessment throughout the study duration.
Statistical Analysis
Data were analyzed with SAS version 9.4 (SAS Institute, Cary, NC). Efficacy data including pseudarthrosis rates and patient-reported outcomes used data from the 142 patients who completed the 12-month postoperative assessment. Safety data were collected from all enrolled patients. Counts and percentages are reported for categorical baseline variables, and the mean, SD, and range are reported for continuous variables. Pre- and postoperative patient-reported outcomes were compared with a Wilcoxon signed rank test. Correlation of outcomes to risk factors was calculated by χ2 test or by Fisher’s exact test if a count was less than 5. Alpha was set at 0.05, and a P value ≤0.05 was considered significant. Figures are presented with error bars showing the SEM unless otherwise noted.
Results
Participants
A total of 263 patients were screened, of whom 224 met the criteria for eligibility and were enrolled into the study. Eighty-two patients did not complete the 12-month follow-up visit; 23 (28.0%) withdrew consent, 11 (13.4%) no longer met the inclusion/exclusion criteria, 3 (3.7%) were noncompliant, 3 (3.7%) were withdrawn by the investigator, 2 (2.4%) had their clinical site close prior to study completion, 1 (1.2%) died (not related to device or procedure), and 1 (1.2%) moved out of the country. A total of 38 patients (16.9%) were lost to follow-up.
A total of 142 patients were available for fusion assessment at 12 months. The mean age was 63.3 ± 10.3 (range 30–81) years and included 79 (55.6%) women. Patients had a mean BMI of 31.0 ± 5.9 (range 17.2–44.2) kg/m2 (Table 1). More than half of participants (55.6%, n = 79) had a BMI ≥30. Additionally, more than half of the participants (54.9%, n = 78) had a single risk factor and the remaining participants (45.1%, n = 65) presented with 2 or more risk factors for pseudarthrosis (Table 2). The majority of participants had 2 levels fused (n = 71; 50.0%), while others had a single level (n = 10; 7.0%) or 3+ levels fused (n = 61; 43.0%).
Demographics and baseline characteristics of study participants (N = 142).
Number of risk factors or fusion levels (N = 142).
Efficacy Assessment
Fusion Rate
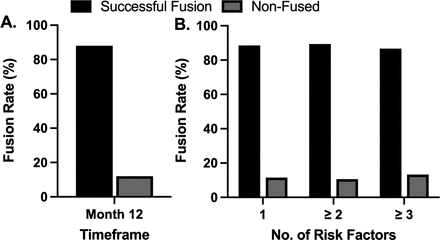
Of the 142 patients evaluated at the 12-month follow-up visit, 88.0% (n = 125/142) were graded as fused (all levels) with 12.0% (n = 17/142) graded as nonfused (Figure 1A). Fusion success was 88.5% for patients with a single risk factor, 87.5% for patients with ≥2 risk factors, and 82.3% for patients with ≥3 risk factors (Figure 1B).
Fusion success at 12 months following pulsed electromagnetic field stimulation. (A) An overall successful fusion rate of 88.0% (n = 125/142) was observed following lumbar spinal fusion with adjunct pulsed electromagnetic field stimulation treatment. (B) A high rate of successful fusion was observed in patients presenting with single or multiple risk factors for pseudarthrosis.
Risk Factors
Risk factors evaluated included nicotine use, diabetes, osteoporosis, prior failed fusion, multiple levels (surgery), and multiple risk factors. Exploratory risk factors included BMI and age. Approximately 54.9% of patients had 1 risk factor, 45.1% had ≥2 risk factors, 12.0% had ≥3 risk factors, and 1.4% had ≥4 risk factors (Table 2). None of the risk factors significantly impacted the proportion of patients who had successful fusion (P > 0.05; Table 3). That is, regardless of known risk factors, similar fusion rates were achieved for patients in the study.
Impact of patient risk factors on fusion success.
Patient-Reported Outcomes
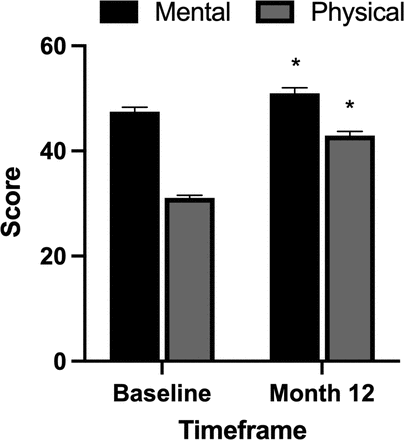
Significant improvements in the SF-36 mental component score and physical component score were observed at the 12-month time point. The overall preoperative mean mental component score was 47.5 ± 10.3 and improved to 51.0 ± 12..6 (P = 0.005) at 12 months. The overall preoperative mean physical component score was 31.1 ± 5.6 and improved to 43.0 ± 9.8 (P < 0.001) at 12 months (Figure 2).
Impact of pulsed electromagnetic field stimulation on short form-36 mental and physical component scores. Significant improvements in short form-36 mental (P = 0.005) and physical (P < 0.001) component scores were observed at the 12-month time point.
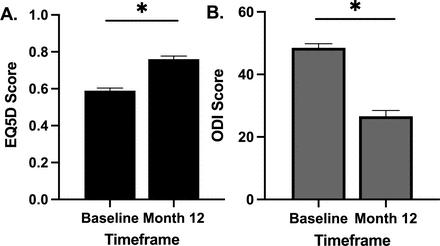
A significant improvement in EQ-5D was observed at the 12-month time point. The overall preoperative mean EQ-5D score was 0.59 ± 0.15 and improved to 0.76 ± 0.17 (P < 0.0001) at 12 months. A significant improvement in ODI was observed at the 12-month time point. The overall preoperative mean ODI score was 48.5 ± 13.9 and improved to 26.6 ± 18.5 (P < 0.0001) at 12 months (Figure 3).
Impact of pulsed electromagnetic field stimulation on overall quality of life and disability index (EQ-5D). (A) A significant improvement in EQ-5D scores was observed at the 12-month time point (P < 0.0001). (B) A significant improvement in Oswestry Disability Index (ODI) scores was observed at the 12-month time point (P < 0.0001).
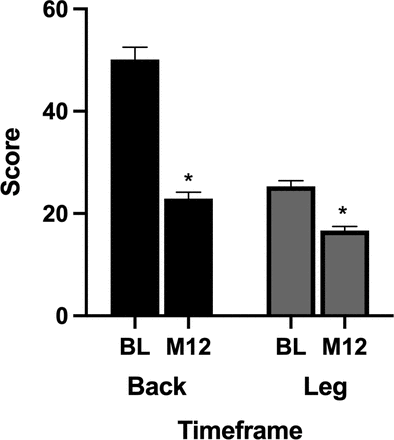
A significant improvement in VAS-back pain score was observed at the 12-month time point. The overall preoperative mean VAS-back pain score was 50.1 ± 24.9 and improved to 22.9 ± 11.7 (P < 0.0001) at 12 months. A significant improvement in VAS-leg pain score was observed at the 12-month time point. The overall preoperative mean VAS-leg pain score was 25.3 ± 7.9 and improved to 16.7 ± 7.4 (P < 0.0001) at 12 months (Figure 4).
Impact of pulsed electromagnetic field stimulation on overall visual analog scale (VAS)-back and VAS-leg pain scores. A significant improvement in VAS-back and VAS-leg pain scores was observed at the 12-month time point (P < 0.0001).
Safety Assessment
A total of 553 adverse events (AEs) were reported over the 12-month period (Table 4). The majority of AEs were considered mild (n = 312, 56.3%), followed by moderate (n = 170, 30.7%) and severe (n = 71, 12.8%). A total of 6 AEs (1.1%) were considered related, and 547 AEs (98.7%) were considered unrelated to the PEMF intervention with the SpinalStim device. Specifics on the related AEs are provided in Table 4.
Safety outcomes.
Discussion
The current study aimed to evaluate the utility of PEMF as an adjunct therapy for lumbar spinal fusion in patients with risk factors for pseudarthrosis. A spinal fusion rate of 88.0% and significant improvements in all patient-reported outcomes was observed at the 12-month postoperative follow-up. These results suggest that adjunctive use of PEMF may be beneficial in lumbar spine surgery patients with known risk factors associated with pseudarthrosis. Successful fusion rates were obtained in this challenging population. A myriad of patient complications and consequences are observed with unsuccessful fusion. These include the need for revision surgery, worsening of patient-reported outcomes, development of new pain or recurrent symptoms, further degeneration, and an increase in financial costs associated with care.37
Evidence exists to demonstrate a lower fusion rate or increased complications profile in patients who are undergoing spinal surgery and present with risk factors for pseudarthrosis.38–40 Pseudarthrosis is a leading cause of postoperative pain and accounts for 45% to 56% of revision surgeries.41 Diabetic patients and those who are currently or have a history of smoking show reductions in fusion rates following spinal surgeries. Glassman et al show a reduced fusion success rate (74%–78% fusion success) in diabetic patients compared with nondiabetic patients (95% fusion success) with increased complications with multilevel fusion.42 Multilevel fusion is a risk factor for pseudarthrosis with each additional level of fusion required shown to reduce successful spinal fusion by ~20%.43 Patients who are current smokers show reduced rates of fusion postoperation compared with nonsmokers.44–46 One study showed pseudarthrosis 1 to 2 years postsurgery in 40% of smokers vs 8% in nonsmokers.44 The current study results show no impact of known risk factors on fusion rate or patient-reported outcomes when treated with PEMF. These findings suggest that PEMF may be a positive addition to postsurgical interventions to improve fusion and outcomes success in patients with risk factors.
Of the risk factors analyzed (ie, nicotine use, diabetes, osteoporosis, prior failed fusion, multiple levels [surgery], multiple risk factors, BMI, and age), no risk factor was significantly associated with a difference in the number of patients who had successful fusion vs those who had failed fusion. The fusion rates in this study are consistent with fusion rates reported in the literature in similar indications using PEMF. Collective results from randomized controlled trials, which compared fusion rates of patients who received PEMF stimulation therapy, showed improved fusion rates compared with control.7,47,48 A review of PEMF fusion rates in spine surgery studies reported a success range of 64% to 98% from controlled, retrospective, and case series studies. Patients who received PEMF as an adjunct to spinal surgery had higher fusion rates than sham, placebo, or no stimulation control groups. In studies comparing PEMF therapy to a control group, fusion rates ranged from 64.0% to 98.0% with PEMF and 43.0% to 87.0% for controls.14
While the primary goal of PEMF after spinal surgery is to facilitate arthrodesis, fusion success is not the only factor that impacts successful patient outcomes. Evaluation of various patient-reported outcomes is of interest in determining the full benefit of any therapeutic intervention. While the patients enrolled were at risk for higher complications, the patients in this study achieved significant improvements in patient-reported outcomes scores that measure disability, function, pain, quality of life, and overall well-being. These findings match evidence from other studies that have reported on similar patient populations with PEMF therapy. Patients with cervical disc herniation, osteoarthritis, and chronic neck/back pain show significant improvement in pain, muscle spasm, joint movement, disability, and quality of life scores after PEMF treatment.11,31,49
Study limitations included potential variability in the determination of fusion status via x-ray/CT imaging and clinical impression per each incidence and standard of care. The study showed a lack of a control arm, which impedes the direct comparison of fusion success and patient-reported outcomes in patients who were treated with PEMF vs without PEMF. In lieu of no control arm, the reported rates serve as an initial basis for our exploration into the benefit of PEMF using SpinalStim following lumbar spinal surgery and provide rates in keeping with other reported clinical evidence. Another study is currently underway to further understand the impact of PEMF use compared with untreated control patients.
Conclusion
Results of the present study add to the body of evidence highlighting PEMF as a positive adjunct tool to aid in bone fusion healing and reduce patient complications, especially in patient populations at risk. PEMF may provide benefit to patients with various individual and/or a combination of risk factors that traditionally contribute to lower fusion rates and worse outcomes overall. PEMF therapy following lumber spinal surgery can be easily integrated into postoperative care and safely used in the home setting in addition to other modalities to improve patient status and success.
Acknowledgments
We thank Stephanie E. Tedford, PhD, of Pharmacologics, Inc, who, on behalf of Orthofix US LLC, assisted in the implementation of author revisions and Deanna Naomi Schreiber-Gregory, MS, who served as the biostatistician for data analysis.
Footnotes
Funding This study was supported by Orthofix US LLC (Lewisville, TX, USA).
Declaration of Conflicting Interests All authors have received financial support from Orthofix US LLC for their involvement in the study. Orthofix US LLC was involved in the design of the study, analyses, and interpretation of data and in writing of the manuscript. In addition, Ilyas Aleem reports receiving consulting fees from Globus. Peter G. Campbell reports receiving payment/honoraria from Stryker (speakers’ bureau) and serves as a consultant for Nexus Spine. Dom Coric reports royalties/licenses and consulting fees from Medtronic, Globus/Nuvasive, and SpineWave and stock/stock options from SpineWave and Premia. Vikas Patel reports grants/contracts from Mainstay Medical, Medicrea, Medtronic, Orthofix, Pfizer, and Spinal Kinetics; consulting fees from Spine Welding and SI Bone; and payment/honoraria from Mainstay Medical. Kristen Radcliff reports consulting fees from Orthofix, 4 web, and Stryker and stock/stock options from 4web and Orthoson. Amer Vokshoor reports research contracts from Spine Art, Orthofix, and Synergy and royalties/licenses from Globus Medical. Joshua J. Wind reports payment/honoraria from Orthofix (speaker’s fee). Hamid Hassanzadeh reports consulting fees from Nuvasive, Medtronic, DePuy, and Orthofix; payment/honoraria from Pfizer and Nuvasive; support for attending meetings from Nuvasive; and a leadership role at the Scoliosis Research Society.
Institutional Review Board Statement The study was conducted in accordance with the Declaration of Helsinki and approved by the Central Western Institutional Review Board (IRB) and local IRBs where applicable (at the Aspirus Research Institute, University of Virginia, and Rothman Institute).
Data Availability Statement Data supporting the findings of this study are available upon reasonable request.
Patient Consent Informed consent was obtained from all patients involved in the study.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2023 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.