ABSTRACT
Background The purpose of our study was to examine the effect of controlled delivery of TGF-β3, BMP-4, and TIMP-2 with a biocompatible biopolymer, chitosan, on an acutely injured intervertebral disc (IVD) in a rabbit model.
Methods After conducting an in vitro analysis of the chondrogenic capacity of the biomolecule cocktail use (ie, TGF-β3, BMP-4, and TIMP-2) and confirming stem cell viability in chitosan hydrogel, 15 New Zealand white rabbits underwent a lateral approach of the L1 to L4 IVDs. In each rabbit, the L2 to L3 IVD was left pristine, whereas the L1 to L2 and the L3 to L4 IVDs in each rabbit underwent nucleotomy via a 25-G needle, and the animal was subsequently randomized to no further treatment (defect only), chitosan alone, Chitosan + TGF-β3 + BMP-4, or chitosan + TGF-β3 + BMP-4 + TIMP-2. At 6 weeks after injury and intervention, the rabbits were killed and spines harvested to undergo quantitative T2 magnetic resonance imaging (MRI) and subsequent histologic analysis.
Results In the in vitro analysis, cells treated with experimental media containing TGF-β3, BMP-4, and TIMP-2 exhibited staining indicative of GAG production and began to exhibit a chondrocytic morphology. Quantitative T2 MRI mapping demonstrates that discs treated with chitosan, chitosan containing TGF-β3 and BMP-4, or chitosan containing TGF-β3, BMP-4, and TIMP-2 had consistently higher T2 relaxation times compared with defect-only discs. When the T2 relaxation times of each treatment group and defect-only discs were normalized to the healthy control disc, it was found that the T2 relaxation time of discs treated with chitosan containing TGF-β3 and BMP-4 and discs treated with chitosan containing TGF-β3, BMP-4, and TIMP-2 were significantly greater compared with defect-only discs (P = .048 and P = .013, respectively). Histologically, animals that received chitosan only, or chitosan with TGF-β3 and BMP-4, showed a significantly higher intensity of Safranin-O staining (P = .016 and P = .02, respectively) compared with control discs, whereas the difference in staining intensity in animals that received chitosan loaded with TGF-β3, BMP-4, and TIMP-2 failed to achieve significance (P = .161).
Conclusions A combination of chitosan, TGF-β3, and BMP-4 was effective at promoting regeneration in an acute disc injury rabbit model, whereas TIMP-2 did not have a significant effect.
INTRODUCTION
The intervertebral disc (IVD) is a complex tissue that serves an important purpose in the biomechanical stability and functionality of the axial skeleton. The disc comprises a gelatinous core called the nucleus pulposus (NP), which is surrounded by the lamellar, fibrocartilagenous annulus fibrosus (AF). The end plates of the vertebral bodies form the inferior and superior boundaries of the disc. The cellular constituents of the NP possess a phenotype that is similar to chondrocytes within articular cartilage, whereas cells from the AF have been described as fibrochondrocytes. NP chondrocytes thrive in a gelatinous, 3-dimensional, amorphous environment, whereas AF fibrochondrocytes respond to the oriented, lamellar, nearly 2-dimensional structure provided by the concentric rings of the annulus. Because of these differences in cell phenotype and structure of the constituents of the disc, healing after injury has been a significant challenge.
Tissue engineering–based strategies, which rely on the use of scaffold materials, multipotent cells, and biomolecules, have been implemented in an attempt to regenerate the IVD.1,2 From a scaffold perspective, hydrogel-based materials offer a promising biomimetic solution to regenerating NP tissue. Hydrogels are polymers that swell in response to various environmental stimuli, and many possess physical properties conducive to injection and subsequently facilitating the 3-dimensional proliferation of cells. Chitosan, which is a biopolymer based on the protein chitin found in crustacean shells, can be formulated to have gel-like properties. Chitosan and chitosan blends have been posited as materials that would be ideal for disc regeneration.3 The handling characteristics of the material can be easily tailored to suit clinical implementations (ie, injection). Additionally, the functional amine groups associated with chitosan interact with other biomolecules, which can be used to tailor drug delivery kinetics. We hypothesize that chitosan hydrogels can be used in the setting of IVD regeneration in terms of both providing an environment conducive to cell proliferation and growth, and serving as a vehicle for the in vivo delivery of cells and growth factors.
Although hydrogels may be the material of choice for scaffolds, the combination of molecular signals necessary for de novo disc tissue formation have not yet been fully elucidated. Our group has examined the effect of a combination of anabolic growth factors and an inhibitor of proteases on an in vitro culture of articular chondrocytes.4 Results indicated that a combination of transforming growth factor β-3 (TGF-β3), bone morphogenetic protein-4 (BMP-4), and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) was effective in increasing extracellular matrix production of these cells. Given the similarities in phenotype between articular chondrocytes and NP cells, an animal study was undertaken in an attempt to translate these results to a relevant animal model. The purpose of our study was to examine the effect of controlled delivery of TGF-β3, BMP-4, and TIMP-2 with a biocompatible biopolymer, chitosan, on an acutely injured IVD in a rabbit model.
MATERIALS AND METHODS
In Vitro Analysis of Chondrogenic Capacity of Biomolecule Cocktail
Four mature New Zealand white rabbits were killed with pentorbital overdose. Bilateral femurs and tibias were harvested using gross dissection, and bone marrow was harvested from each intermedullary cavity. The bone marrow was immediately washed and immersed in basal medium (Dulbecco modified Eagle medium [DMEM], 10% fetal bovine serum, 1% Pen/Strep) on ice. An average of 10 to 12 mL of bone marrow was obtained per animal. A red blood cell lysis was performed using 30 mL of red blood cell lysis solution for 10 minutes at room temperature with frequent agitation. The resulting bone marrow cell solution was plated on T75 flasks to allow for the attachment of plastic-adherent cells. All flasks were washed 3 times with sterile phosphate-buffered saline after 24 hours to remove nonadherent cells, and adherent cells were visualized. This method has been previously shown to yield a population of multipotent cells able to maintain capacity of tri-lineage differentiation for multiple passages.5,6
Cells from the second passage (P2) exhibited a fibroblastic appearance and formed multiple high-density fibroblastoid colonies in monolayer culture. P2 cells were plated at 25 000 cells per well in a 24-well plate. Chondrogenic differentiation was assessed in chondrogenic medium (DMEM, 1.25 mg/mL bovine serum albumin, 1% fungizone, 1 mM sodium pyruvate, 0.1 mM ascorbic acid, 10−8 M dexamethasone, and 10 ng/mL TGF-β3). Furthermore, experimental media consisting of DMEM (with 1% fetal bovine serum and 1% fungizone) and supplemented with a combination of anabolic growth factors (100 ng/mL TGF-β3, 100 ng/mL BMP-4) and a catabolic inhibitor (100 ng/mL TIMP-2) was also assessed. These concentrations were selected based on our previous in vitro work that showed promising anabolic responses.4 Control cells were treated with basal medium. After exposure to the different media compositions, chondrogenic differentiation of isolated marrow cells was assessed by fixing the cells in 10% neutral buffered formalin after 14 or 21 days in culture, and staining with 1% Alcian blue solution for 30 minutes. All wells were rinsed 3 times with 70% EtOH before being imaged using light microscopy (IX-71; Olympus Inc, Waltham, MA) to quantify staining intensity. All experiments were performed in n = 6 wells per group per time point. Alcian blue staining intensities were calculated for each experimental well and compared to control cells.
Synthesis of Chitosan Hydrogel and Effect on Stem Cell Viability
In order to characterize the effect of the chitosan hydrogel on the viability of resident cells, cells isolated from rabbit bone marrow were cultured in a chitosan hydrogel. The hydrogel consisted of 3 wt % chitosan (pH = 6.5), which was dissolved in 0.5% acetic acid at 4°C for 48 hours. Dissolved chitosan was mixed with 0.025 g/mL ammonium hydrogen phosphate. This resulted in physiologic pH equilibration (pH = 7.4), cross-linking of chitosan molecules, and subsequent gelation into a viscous gel at 37°C. At 4°C, the hydrogel remained a viscous, free-flowing solution, which enabled the homogenous mixing of 105 bone marrow cells per 1.0 mL of the cross-linked Ch solution, which was subsequently incubated at 37°C to allow for gelation. After 30 minutes, 1.5 mL of basal medium was added to each well containing the cross-linked chitosan/marrow cell mixture. New basal medium was replenished every third day. After 14 days of culture, media was aspirated from each well, and 1.5 mL of a live/dead staining solution was added to each well to quantify the fraction of viable cells. Following live/dead staining, 3-dimensional laser confocal microscopy was used to image a 250-μm tall stack (5-μm step size) of each well to obtain a 3-dimensional volume of stained cells within the chitosan matrix.
Rabbit Nucleotomy Model and Treatment with Biomolecule-Loaded Chitosan Hydrogels
Fifteen female New Zealand white rabbits (2.8–3.2 kg) were used in accordance with a protocol approved by our Institutional Animal Use and Care Committee. Animals were anesthetized via intramuscular ketamine/xylazine, intubated, and maintained with inhaled isoflurane in a lateral position on the surgical table. After shaving a sterile prepping surgical site, a longitudinal midline incision was made over the lumbar spine, followed by a single fascial incision lateral to midline. Blunt dissection was employed to facilitate visualization of the IVDs at L1 to L2, L2 to L3, and L3 to L4, which were also confirmed with fluoroscopy. A 25-G spinal needle was then inserted into the nucleus of the L1 to L2 and the L3 to L4 discs, while keeping the L2 to L3 disc pristine. The center-center position of the needle within the nucleus was verified on multiple fluoroscopic views. Following confirmation of positioning, the disc was aspirated using a vacuum. The aspirated discs were then randomized to receive no further intervention (defect only), or treatment with chitosan, as shown in Table 1.
Treatment groups used in experimentation.
Hydrogels consisted of 3 wt % chitosan cross-linked with ammonium hydrogen phosphate, and were synthesized as described for the previous injection. After cross-linking, biomolecules were homogenously mixed into the solution at a concentration of 100 ng/mL each. Hydrogels were maintained at 4°C to facilitate injection into the disc space through the inserted 25-G needle. Treated discs received either chitosan alone, chitosan loaded with either TGF-β3 and BMP-4, or chitosan loaded with TGF-β3, BMP-4, and TIMP-2. Injection volumes remained consistent (15 μL) regardless of the inclusion of biomolecules. Hydrogels were synthesized within 24 hours of injection.
After treatment of the discs, fascial and skin incisions were closed separately and animals were recovered from anesthesia. Animals were allowed ad libitum activity for a period of six weeks after surgery with free access to food and drink. At the sixth postoperative week, rabbits were anesthetized with intramuscular ketamine/xylazine and then administered an overdose of pentabarbitol. Lumbar spines were aseptically harvested and stored in zinc-buffered 10% formalin until histologic processing.
Quantitative T2 Magnetic Resonance Imaging
Following necropsy and en bloc excision of the lumbar spine of each rabbit, quantitative T2 mapping was performed. This magnetic resonance imaging (MRI) modality has been previously shown to correlate to proteoglycan content of the nucleus pulposus and has been demonstrated to correlate to the degree of IVD degeneration.7,8 Lumbar spines were placed in a 6-channel send-receive wrist coil in a 3-T magnetic resonance imaging system. Following coronal and axial localizer sequences, multislice, multiecho T2-weighted maps were obtained. Coronal maps were obtained with TR = 2000, and TE = 11.1 ms, 22.2 ms, 33.3 ms, 44.4 ms, and 55.5 ms, with a 2-mm slice thickness (0.2-mm slice gap), 180° flip angle, and 6 averages in a 140 × 50 pixel matrix (signal-to-noise ratio [SNR] = 1.0) Axial maps of each disc were obtained with TR = 1500, and TE = 12.2 ms, 24.4 ms, 36.6 ms, 48.8 ms, and 61.0 ms, with a 2-mm slice thickness (0.2-mm slice gap), 180° flip angle, and 2 averages in a 102 × 34 pixel matrix (SNR = 1.0). High-resolution T2 turbo spin-echo (TSE) sequences were acquired for gross visualization. T2 mapping was performed by curve fitting the logarithm of the signal intensity to echo time to obtain the quantitative T2 value in a pixel-by-pixel manner:

Parametric T2 maps were analyzed using region of interest processing. Axial maps of each disc (control, defect-only, and treatment) were used to quantify the mean T2 relaxation time of the NP and of the entire IVD. Analysis was performed in triplicate in a blinded fashion.
Histologic Analysis
Following MRI scans, the spines were then prepared for decalcified histology. The spines were kept intact during processing, such that the L1 to L2, L2 to L3, and L3 to L4 discs could be visualized simultaneously during microscopic characterization. Decalcified sagittal thin sections were stained with Safranin-O/Fast Green to aid in the quantification of proteoglycan content and distribution. Slides were digitized using an automated slide scanner at a magnification of ×20. A custom-written MatLab algorithm was used to quantify Safranin-O staining intensity and total disc size. Three slides per animal, each containing an untreated nucleotomized, healthy, and treated nucleotomized disc, were also assessed by a blinded observer. The extent of IVD degeneration was qualitatively assessed using the grading scheme developed by Rutges et al.9
RESULTS
In Vitro Bone Marrow Stromal Cell Differentiation
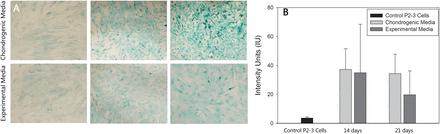
Cells treated with differentiation media showed significantly greater staining intensity than control cells (Figure 1A). Furthermore, cells treated with experimental media containing TGF-β3, BMP-4, and TIMP-2 exhibited staining indicative of GAG production and began to exhibit a chondrocytic morphology, suggesting chondrogenic differentiation due to treatment with the aforementioned growth factors and the proteinase inhibitor TIMP-2. Quantitative image analysis of staining intensity demonstrated that cells treated with chondrogenic media exhibited significantly greater Alcian blue staining intensity, respectively (Figure 1B).
Quantitative results of differentiation assays. (A) Both chondrogenic media and experimental media containing TGF-β3, BMP-4, and TIMP-2 induced time-dependent increases in Alcian blue staining. (B) There was significantly greater staining intensity compared with control P2-3 cells treated with basal media.
Quantitative T2 MRI
Quantitative T2 MRI mapping demonstrates that discs treated with chitosan, chitosan containing TGF-β3 and BMP-4, or chitosan containing TGF-β3, BMP-4, and TIMP-2 consistently had consistently higher T2 relaxation times compared with defect-only discs. Furthermore, T2 mapping was sensitive to the increased proteoglycan content in the NP, because the mean T2 relaxation time was consistently higher in the NP compared with the whole disc.
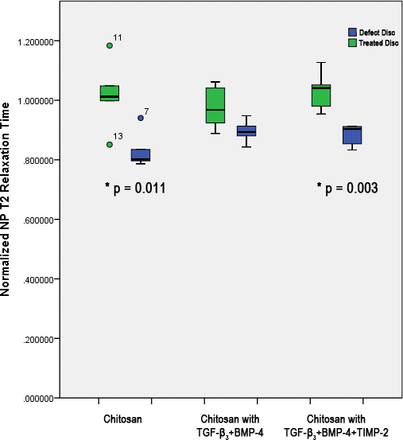
When T2 relaxation time of each treatment group and defect-only discs were normalized to the healthy control disc, it was found that the whole-disc T2 relaxation times of discs treated with chitosan containing TGF-β3 and BMP-4 and discs treated with chitosan containing TGF-β3, BMP-4, and TIMP-2 were significantly greater compared with defect-only discs (P = .048 and P = .013, respectively). Discs treated with chitosan only did not exhibit a difference in normalized T2 relaxation time compared with defect-only discs. When only the NP was analyzed, it was found that only discs treated with chitosan only and discs treated with chitosan containing TGF-β3, BMP-4, and TIMP-2 exhibited greater normalized T2 relaxation time compared with defect-only discs (P = .011 and P = .003, respectively; Figure 2).
Normalized nucleus pulposus (NP)-only T2 relaxation times of defect-only discs, and discs treated with chitosan alone, chitosan containing TGF-β3 and BMP-4, or chitosan containing TGF-β3, BMP-4 and TIMP-2. *Significant difference between treated and defect-only discs at P < .05.
Histologic Analysis
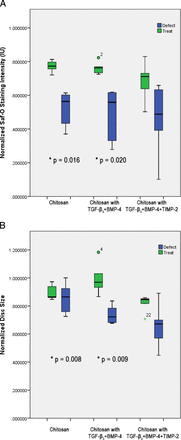
Quantitative analysis of Safranin-O staining, which is proportional to sulfated glycosaminoglycan content, demonstrated higher intensity staining in all treated discs compared with those that received a defect without treatment (Figure 3A). Animals that received chitosan only or chitosan with TGF-β3 and BMP-4 showed significantly higher intensity staining (P = .016 and P = .02, respectively) compared with their internal control defect discs, whereas the difference in staining intensity in animals that received chitosan loaded with TGF-β3, BMP-4, and TIMP-2 failed to achieve significance (P = .161). Semiautomated analysis of total disc area (Figure 3B) also demonstrated that discs treated with chitosan or chitosan with TGF-β3 and BMP-4 were significantly larger than their respective internal control defect-only discs (P = .008 and P = .009, respectively).
Quantitative analysis of Safranin-O staining intensity (A) and total disc size (B) from Safranin-O/Fast Green–stained decalcified sections. *Significant difference between treatment and defect discs.
Qualitative analysis of histologic sections using the Rutges classification9 showed that a healthy disc had an average total score of 1.89 ± 1.45, whereas the average total score for defect-only discs ranged from 3.91 ± 2.74 to 4.46 ± 2.73 (Table 2). Although average total scores for each of the treatment discs were lower than their respective defect-only discs, none achieved a statistically significant difference. Discs treated with chitosan alone achieved a significantly better score on nucleus pulposus matrix organization (P = .02) and nucleus matrix staining (P = .046) compared with the internal defect-only discs. Animals that had discs treated with chitosan loaded with TGF-β3 and BMP-4 displayed significantly better annulus fibrosus organization (P = .005) and nucleus matrix staining (P = .017) compared with defect discs.
Results of qualitative grading of Safranin-O/Fast Green slides according to the system developed by Rutges et al.9a
No adverse or allergic reaction was noted by gross examination, MRI, or histology.
DISCUSSION
In general, degenerative disc disease is considered to be progressive and irreversible. Given its prevalence in the general population and its role as a public health concern, the regeneration of the IVD is of high interest among scientists and clinicians. However, it remains a lofty goal in which there are many technical challenges. In theory, attempts at a solution to this challenge combine a scaffold, growth factors, and multipotent cells to create the structural, signaling, and biologic environment for IVD regeneration. In our study, we examined the use of anabolic growth factors (TGF-β3 and BMP-4) and a protease inhibitor (TIMP-2), in combination with a chitosan scaffold-based delivery mechanism in the setting of a rabbit acute lumbar disc injury model. We found that, compared with injured discs without intervention, TGF-β3 and BMP-4 with a chitosan scaffold, as well as TGF-β3, BMP-4, and TIMP-2 with a chitosan scaffold, were able to limit progression of proteoglycan loss and imaging characteristics of IVD degeneration. Histologically, a chitosan scaffold along with TGF-β3 and BMP-4 displayed significantly better annulus fibrosis organization and nucleus matrix staining.
Our study is limited by our use of an acute disc injury model, as opposed to a more chronic model of IVD degeneration. Disc injury was created at the time of injection, which creates a reliable and reproducible model by which the effects of our intervention could be examined. However, an acute model has limited clinical translation, because most patients will present to the treating clinician after a significant amount of disc space loss, inflammation, and proteoglycan loss has already occurred in the IVD. However, for the purposes of initial evaluation of the effect our scaffold and growth factor combination, an acute model provided a reliable and reproducible method to examine its effects. Additionally, our exploratory study was limited by low sample size. Future studies should focus on higher group sizes to increase statistical power.
The search for the ideal scaffold to restore the biomechanical properties of the NP, as well as provide a suitable local environment for IVD regeneration, is ongoing. In our study we used chitosan, a biopolymer that has shown promise as a carrier for IVD regeneration. There is evidence that oxidative stress and subsequent apoptosis are responsible for some portion of IVD degeneration.10 Chitosan may provide protection from this phenomenon for NP cells, making it a suitable carrier and supplement for regenerative therapies.11,12 In addition, an ideal carrier should provide a suitable environment for cellular growth and differentiation. Although outside the scope of our current study, bone marrow–derived stem cells have had positive effects in the treatment of degenerative disc disease in both preclinical and clinical settings, and as such, the scaffold used in IVD regenerative treatment must be able to provide a suitable environment for the growth and differentiation of these cells.13–15 Chitosan has shown the ability to provide an appropriate medium for mesenchymal stem cell differentiation.16 Chitosan-based nanoparticles may also assist in the reduction of inflammation associated with disc damage.17 Lastly, the scaffold used in regenerative therapies must exhibit the mechanical properties of the NP to assist in its physiologic function as a “shock-absorber.” Showalter and colleagues18 have demonstrated favorable biomechanical properties of a chitosan-based hydrogel in a human cadaveric model. In addition, Gullbrand et al19 have shown favorable mechanical properties of chitosan-based hydrogel in a goat preclinical model.
In our study, when analyzing only the NP, we found that chitosan-only discs exhibited greater normalized T2 relaxation time compared with defect-only discs. This suggests that even introduction of the mechanical and microenvironment support by the chitosan hydrogel itself helps to prevent continued degeneration, possibly for the reasons described previously. Although chitosan-based hydrogels have shown promise, other options have been described and explored. Gellan gum–based hydrogels, self-assembling peptidic hydrogels, hyaluronic acid–based gels, and fibrin gels have all been used in preclinical models.20–22
Altered growth factors play an intimate role in the development of degenerative disc disease. As such, regenerative therapies for IVD degeneration must aim to correct these changes. Because these alterations are likely local changes and not necessarily systemic changes, correction of unfavorable growth factor abnormalities may need to be delivered directly to the disc.23
TGF-β is a family of anabolic growth factors that plays an important role in differentiation of mesenchymal stem cells into a chondrocytic lineage. Specifically, TGF-β3 has been used to induce IVD-like cells in an in vitro setting, as well as to support AF matrix formation.24,25 A combination of TGF-β3 and BMP-4 has been used to form cartilage tissue in an in vitro model.26 Our study was able to show increased T2 relaxation time in discs treated with TGF-β3 and BMP-4 compared with defect-only discs, implying that these anabolic growth factors prevented degeneration to some degree. Histologically, this combination assisted with increasing annulus fibrosis organization.
Matrix metalloproteinase-2 (MMP-2) and TIMP-2 sit on the see-saw of extracellular matrix degradation and protection via inhibition, respectively.27 MMP-2 has been shown to be overexpressed in the setting of disc injury.28 In patients with cervical disc herniation, MMP-2 levels are increased, whereas TIMP-2 levels are decreased.29 Our study found that the addition of TIMP-2 to a chitosan scaffold and anabolic growth factors were effective at reducing disc degeneration compared with a defect-only disc.
Chitosan alone, as well as chitosan plus anabolic growth factors, was effective at reducing degeneration after an acute disc injury in a rabbit model. We did not find that the addition of TIMP-2 was helpful at reducing degeneration in our study. Future studies should examine the effect of these interventions in a chronic disc disease model that more closely resembles human pathology. In addition, the addition of mesenchymal stem cells to the growth factor and scaffold interventions may provide a more complete regenerative approach, and should be the target of future studies
ACKNOWLEDGMENT
This manuscript is dedicated to the memory of Harry N. Herkowitz, MD, chairman of the Department of Orthopaedic Surgery at Beaumont Health from 1991 to 2013.
Footnotes
Disclosures and COI: This research was funded by the Department of Orthopaedic Surgery at Beaumont Health. The authors declare no conflicts of interest related to the subject matter of this manuscript. All authors approved and take responsibility for the content of the manuscript.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2020 ISASS.