Abstract
Background Anterior vertebral body tethering (AVBT) has been approved for skeletally immature (IM) adolescent idiopathic scoliosis patients, but the role of AVBT in patients with minimal remaining skeletal growth is controversial. The purpose of this study was to compare minimum 2-year (YR2) outcomes in skeletally IM patients vs those with minimal remaining skeletal growth.
Methods Patients with single thoracic AVBT were grouped by their preoperative (PR) skeletal maturity: IM (n = 16, Risser 0–2) vs mature (M, n = 19, Risser 3–5). Outcomes were assessed at PR, first erect (FE), and YR2. Median (range) was compared with nonparametric tests (P < 0.05).
Results The PR age was 12.5 (9–16) vs 15 (12–18) years with major Cobb 51° (36°–69°) and 49° (40°–69°) for IM and M, respectively. At FE, there was no difference in correction; however, at YR2, the IM group yielded a lower residual curve (15° [−16° to 38°] vs 29° [12°–42°], P = 0.008). Thoracolumbar/lumbar curves were corrected without group differences. Clinically successful correction (<35°) (15 [94%] vs 15 [79%]) and suspected cord breakages (2 [13%] vs 2 [12%]) were similar at YR2. Two overcorrections occurred, both in IM patients. Scoliosis Research Society-22 outcomes at final follow-up were similar between groups. No revision reoperations or conversions to spinal fusion were needed.
Conclusions Skeletally IM patients benefit from greater growth-modulated curve correction than M patients, however, at the increased risk of overcorrection. M patients maintained clinically significant correction at latest follow-up. Longer-term follow-up is required to determine durability of outcomes for patients undergoing AVBT who have minimal remaining growth at the time of index surgery.
Clinical Relevance This study is relevant to spine surgeons, spine physiotherapists, and patients with idiopathic scoliosis. It offers evidence of clinical correction of scoliosis in mature patients.
Level of Evidence 3.
Introduction
Adolescent idiopathic scoliosis (AIS) is the most common pediatric spinal deformity.1–3 Posterior spinal fusion (PSF), the gold standard operative treatment, is associated with potential long-term complications such as degenerative disc disease, back pain, radiculopathy, and loss of mobility.4,5 Anterior vertebral body tethering (AVBT) received US Food and Drug Administration Humanitarian Device Exemption approval in 2019 and has become an alternative to PSF for skeletally immature (IM) patients.5–9 AVBT depends on skeletal growth potential that is harnessed for progressive correction in accordance with the Hueter-Volkmann law,1,2,10,11 as borne out in animal models,12–15finite element analysis,16–18 and clinical case series.5–7,9,19–22 Initial series containing skeletally IM patients (Risser 0–1) have yielded high overcorrection rates19,20 necessitating revision surgery or conversion to PSF.5,8,9 AVBT remains a promising approach,23 and recent evidence suggests delaying the procedure to mitigate against overcorrection.6
AVBT is unproven in skeletally mature (M) patients, given growth modulation cannot occur, but the expansion of indications to those with minimal remaining skeletal growth would provide a treatment alternative to 21% of patients for whom fusion has been the only option.24 To date, most studies have reported on AVBT results in IM patients, and thus evaluation of long-term outcomes comparing IM and M patients is warranted to evaluate ideal indications, timing, as well as the ideal amount of surgical correction vs anticipated growth modulation.5,6,9,19 The senior author gained experience with the M population after patients who initially had significant remaining growth presented months after the initial consultation for surgery. However, the clinical success appeared consistent eventually leading to an expansion of indications. The aim of this study was to compare 2-year (YR2) radiographic and clinical outcomes of thoracic AVBT in patients with AIS who underwent surgery before their skeletal growth acceleration phase vs patients with minimal remaining growth.
Patients and Methods
Following Institutional Review Board approval, a prospectively maintained database was retrospectively reviewed for consecutive AIS patients undergoing AVBT of a thoracic major curvature. All surgeries were conducted by a single surgeon between 2016 and 2018 in patients <18 years old at the time of surgery with a main thoracic curve 40° to 70° and minimum of YR2 follow-up. Indications for surgery in both groups followed standard clinical indications including curve progression and/or the potential for curve progression, clinical deformity, and pain. Skeletal maturity was measured preoperatively and throughout follow-up, according to the ossification of the iliac apophysis (Risser sign) and proximal humeral physis (proximal humerus ossification system [PHOS]). Patients were stratified by Risser, with bone age at or leading up to the growth acceleration phase being designated as skeletally IM (Risser 0–2) vs patients with minimal remaining skeletal growth (Risser 3–5). Left hand x-ray images were not uniformly available to assess Sanders stage for this analysis.25,26
Demographic, Radiographic, and Clinical Outcomes
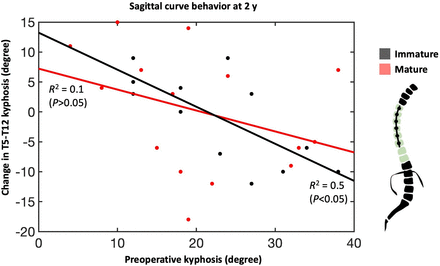
Clinical and radiographic outcomes were collected at the preoperative (PR) visit, first erect (FE), and after YR2. Coronal curves included the instrumented thoracic curvature and noninstrumented compensatory thoracolumbar/lumbar (TL/L) curvature. Sagittal curve behavior was captured by T5-T12 kyphosis. Thoracoplasty was defined as >3 rib resections. Clinically successful correction was quantified by a residual curve <35° at FE and YR2, as defined by Newton et al.19 Height change between upper and lower instrumented levels (UIV-LIV)6 and proportional height gain as a function of body height were calculated. FE% and total YR2% corrections were calculated in relation to PR measurements, whereas follow-up percent correction was calculated between FE and YR2 (Figure 1). Vertebral body wedging was measured as the angle of the apical vertebra’s cephalad and caudad endplate margin on coronal x-ray images comparing FE with YR2, where the difference quantified vertebral body squaring (VBSQ) with more positive values indicating greater squaring.
Sagittal curve behavior, characterized as T5-T12 kyphosis, shows an inverse relationship between preoperative kyphosis and the change in curvature over 2 y. This relationship was statistically significant for immature patients whose spines are more amenable to growth modulation from anterolateral compression.
Complications
All-cause complications were assessed. Overcorrection was defined as a negative YR2 angle and loss of correction as a curve increases >5° between FE and YR2. Screw plowing was defined as the transverse translation of the screw, whereas screw loosening occurred postoperatively.
Surgical Technique
Instrumentation in this series was designed for adult lumbar spinal fusion and used “off label” (Dynesism, Zimmer Biomet). The patient was positioned in the lateral decubitus position. A minithoracotomy and 1 to 2 posterior axillary line portals were made for screw placement and an anterior axillary line portal for scope placement. Thoracoplasty of 3 to 5 ribs for rib prominence was offered if inclinometer measurements were ≥15°. Anterior staples were placed anterior to the rib head at each level, followed by bicortical screw placement, and the tether was then seated in the proximal screw. Under fluoroscopic guidance, sequential compression across each level was applied using a tensioner, and set screws were locked into position. We aimed for residual on-the-table curvature of 15° to 20° for IM patients and maximal correction for M patients. Although, we used similar surgical corrective maneuvers between the IM and M groups, we used a combination of manual force through the vertebral body screws and tensioning to remove slack from the tether maximally in the M group. Double cords with 2 rows of screws were instrumented in patients expected to have less bone remodeling and the need for a more robust composite to withstand forces of activities of daily living, while providing durability of the construct to permit potential bone remodeling.
Statistical Methods
Nonparametric analyses were used due to non-normal data distributions. Categorical and continuous variables were compared between groups using χ 2 test or Mann-Whitney U test, respectively. Within-group comparisons were compared across timepoints using a Mann-Whitney U test with Bonferroni correction (P < 0.05). Data are presented as n (%) or median (range).
Results
A total of 89 patients were treated with AVBT and 35 (39%) underwent only instrumentation of their main thoracic curvature. There were 16 skeletally IM and 19 skeletally M patients. Average final follow-up time was 25.2 ± 3.5 (24–39) months.
Demographic and Skeletal Maturity Measurements
Demographic characteristics were not significantly different between groups (Table 1). Curve distributions were 83% (n = 29) Lenke 1, 14% (n = 5) Lenke 2, and 3% (n = 1) Lenke 3. The age of the IM and M cohorts was 13 (9–16) vs 15 (12–18) years, respectively. At YR2, the skeletal maturity was 4 (1–5) vs 5 (4–5) (Table 2). In the M group, only 3 patients were Risser 4, and the remainder were Risser 5.
Demographics and perioperative results.
Preoperative and follow-up radiographic curve behavior of instrumented major curve, noninstrumented compensatory curve, and T5-T12 kyphosis.
Perioperative Measurements
There was no difference in pre- and postoperative characteristics between groups (Table 1). One cord was instrumented in 16 patients in both groups, whereas 2 cords were used 3 times in the M group (P = 0.09).
Coronal and Sagittal Radiographic Outcomes
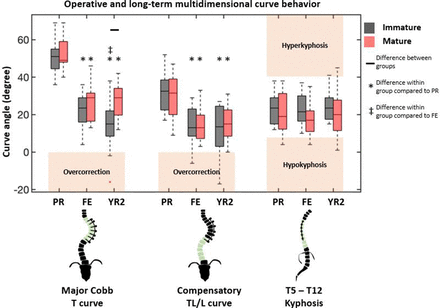
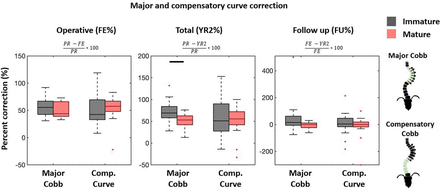
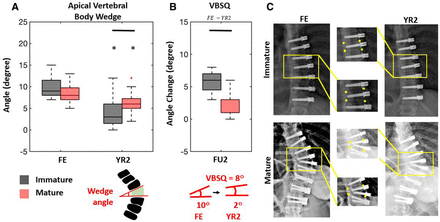
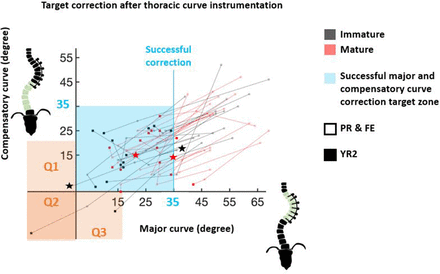
Median PR and FE major curve were similar between groups, both decreasing from 51° (36°–69°) and 49° (40°–69°) to 23.5° (4°–36°) and 29° (13°–46°) in the IM and M group, respectively (Table 2, Figure 2). The YR2 major curve was 15° (16°−38°) vs 29° (12°–42°) (P = 0.008). There was no difference in FE% correction; however, the M group achieved significantly greater YR2% correction (69% vs 53% [P = 0.008]) (Figure 3). Follow-up percent correction was zero for M and 15% for IM patients (P = 0.09). Major curves corrected significantly when compared with PR in both cohorts; however, only the IM group corrected further between FE and YR2 (Figure 2). TL/L compensatory curve measurements were similar between groups at all timepoints (Figures 2 and 3). When data were pooled, there was no difference in T5-T12 kyphosis between groups and within groups at any timepoint (Figure 2). However, subsequent patient-specific analysis showed patients with kyphosis below the mean tended to have an increase in kyphosis and those with kyphosis above the mean tended to have a decrease in their kyphosis (Figure 1) Two (100%) PR hypokyphotic patients became normokyphotic at YR2 and 27 (90%) normokyphotic patients remained normokyphotic. There was a difference in UIV-LIV height at PR and FE, but this difference was not seen at YR2 (Table 3, Figure 4A). UIV-LIV height gain was larger (P < 0.001) for IM patients after YR2; however, groups were similar when normalizing by total body height (Figure 4B). The degree of apical vertebral body wedging was not different at FE but was smaller in the IM group at YR2 (P = 0.02), which leads to greater VBSQ of 6° vs 1° (P < 0.001) (Table 3, Figure 5).
Coronal and sagittal radiographic measurements at 3 timepoints show differences due to skeletal maturity only at 2-y follow-up in the instrumented thoracic curve. Differences between groups are designated by black bar. Differences within a group with reference to preoperative and first erect values are denoted by asterisk (*) and cross (‡), respectively. Boxplot interquartile range shows 25% to 75% with black bar indicating median. Outliers are designated by red plus sign (1.5 times interquartile range). Between- and within-group comparisons were done using a Mann-Whitney U test with Bonferroni correction (P < 0.05). PR, preoperative; FE, first erect; YR2, 2 y; T, thoracic; TL/L, thoracolumbar/lumbar.
Major and compensatory curve first erect and total percent correction (FE% and YR2%) are referenced to preoperative (PR) curve, whereas follow-up percent correction (FU%) compares FE with YR2. Percent correction beyond 100% defines overcorrection, and negative values define loss of correction. Large variations seen in FU% occurs with near-zero FE curves. Boxplots follow formatting described in Figure 2. Between-group comparisons were done using a Mann-Whitney U test with Bonferroni correction (P < 0.05).
Preoperative and follow-up body height, instrumented segment vertical height measurements, and apical vertebral body squaring.
(A) Vertical height changes of the instrumented segment show significant differences between groups at preoperative (PR) and first erect (FE) that disappear at 2 y (YR2). (B) The immature group had greater height gain at YR2, but this difference disappeared when normalizing by total body height gain. Between- and within-group comparisons were done using a Mann-Whitney U test with Bonferroni correction (P < 0.05). UIV-LIV, upper and lower instrumented levels.
(A) The degree of apical vertebral body wedging was assessed at first erect (FE) and compared with the 2-y (YR2) wedge. (B) Vertebral body squaring (VBSQ) quantifies the difference in wedging from FE to YR2, where more positive values indicate greater squaring. Immature patients showed significantly more squaring. (c) Representative immature and mature patients highlight changes in wedging. Between-group comparisons were done using a Mann-Whitney U test with Bonferroni correction (P < 0.05).
Clinical and Patient-Reported Outcomes
Successful major curve correction at YR2 was achieved in 15/16 (94%) and 15/19 (79%) IM and M patients, respectively (Table 2, Figure 6). Of the 5 unsuccessful cases, 2 had successful FE correction and 1 lost correction due to suspected breakage. Only in a Risser 4 patient with a 69° PR curve did the major curve never correct <35°. There was no difference in cord survival and number of suspected cord breakages (Table 4). Breakages occurred at 24, 39, 12, and 43 months, yielding curvature changes of (IM: +8°, −2°; M: +8°, +11°) and only 1 per patient. There were 2 (13%) overcorrections in the IM and 0 in the M and no significant difference in loss of correction. Figure 7 illustrates a case of overcorrection. There was 1 case of screw plowing with angulation >10°, which occurred during operative tensioning of the tether from inertial insertion, but no screw loosening, readmissions, or revisions. There were no significant differences in Scoliosis Research Society-22 outcomes between maturity groups with scores ranging between 3 and 5 for all subsections (Table 4).
Correction of major thoracic (instrumented) and compensatory thoracolumnbar/lumbar (noninstrumented) curves toward target region (light blue, <35°) is captured from preoperative (PR) (unfilled) and first erect (FE) (unfilled) to 2-y (YR2) follow-up (filled). Overcorrection of major (Q1), compensatory (Q3), or both curves (Q2) is highlighted in orange. Suspected breakage is indicated by star.
Complications and patient-reported outcomes.
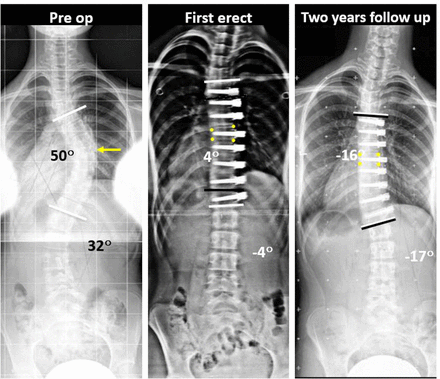
Preoperative posteroanterior scoliosis radiograph of an immature group patient (Risser 2, proximal humerus ossification system 1 [PHOS 1]) with a 50° thoracic curve and 32° thoracolumbar curve. First erect (FE) radiograph shows correction of major and compensatory curves. FE% correction was 92%. The patient was Risser 4 (PHOS 5) at 24 mo, and posteroanterior radiograph shows major curve overcorrection (2-y percent correction [YR2%] = 132%), corresponding to a 500% follow-up percent change (from FE to YR2). Apical wedge angle was 8° at FE and 0° at YR2, generating a vertebral body squaring of 8°. Follow-up angles were measured between vertebra contained by the black bars, where white bars indicate preoperative angles. Yellow arrow indicates the apical vertebra, and yellow dots mark the vertebral body margins.
Discussion
This study compared the effect of PR skeletal maturity on ≥2-year postoperative clinical and radiographic outcomes following thoracic AVBT. Prior literature captures outcomes in IM cohorts8,9,19,23 and only a few report subgroups of slightly more M patients.5,6 To date, there is a singular case series of AVBT in skeletally M patients, but the study reports on a small sample size (N = 10) with less than YR2 follow-up.27 We report on the largest series of patients with limited remaining skeletal growth at the time of surgery and minimum YR2 follow-up.
In our IM cohort (12.5 ± 1.7 years), we observed comparable successful major thoracic curve correction in 94% of patients at YR2, with 2 overcorrections, an 8° correction loss in 1 patient, and no revisions. Samdani et al20 published on 11 IM patients (Risser 0.6 ± 1.1), reporting clinical success (<25°) in all but 1 and 9% to 18% overcorrection. Newton et al19 reported on 17 IM patients (Risser 0, 94% open triradiate cartilage), reporting 59% clinical success, 23% overcorrection, and 4 patients requiring revision surgery due to overcorrection. Miyanji et al9 published on 55 IM patients (Risser 0.5 ± 0.9) showing clinical success in 77%, counting 1 (2%) overcorrection, and 5 (8%) insufficient correction, all of which required conversion to PSF. Alanay et al6 reported on 21 patients spanning the skeletal maturity spectrum, reporting 19% overcorrection (all occurring in Sanders ≤3). Hoernschemeyer et al5 published on 29 patients (48% Risser ≤1) reporting success (<30°) in 20 (75%) patients. The revision rate was 21%, of whom 2 patients converted to PSF due to curve progression.
Our M cohort contained 19 patients (15.4 ± 1.8 years), of whom 15 (79%) were clinically successful at YR2 with 53% correction, a curve progression in 4 (23%), and no overcorrections or revision operations. A small cohort reported by Alanay et al6 was aged 13 ± 1.0 (Sanders 6–7, Risser 4) with 100% success rate (residual curve 9°–27°) at final follow-up (12–29 months) and 55% correction. Hoernschemeyer et al5 report successful correction at last follow-up in 2 (66%) skeletally M patients (Sanders 6–7). Studies of PSF in skeletally M AIS patients with thoracic major curves have shown slightly higher corrections between 65% ± 11%28 and 73% ± 12%.29 This difference may be acceptable for patients with minimal remaining skeletal growth seeking to avoid fusion. Similar patient-reported outcomes between our skeletal maturity groups support the notion that skeletally M patients appear satisfied with the surgical outcome at YR2 removed from surgery. An important future consideration is whether these radiographic outcomes will be durable beyond YR2 when compared with PSF.
Both our IM and M cohorts saw reductions in their major thoracic curves concordant with previously reported literature (51°–23.5° vs 49°–29°), reflecting minimal influence of PR skeletal maturity on surgical curve correction.5,6,9,19,20 Like prior studies that stratified by maturity, we also found greater residual curves in M patients, due to continued correction in the IM group and not widespread loss of correction. M patients maintained correction with no difference in clinically successful correction after YR2 between groups. Overcorrection was only observed in IM patients, which agrees with the literature,9,19,20,22 and confirms prior conclusions that less skeletally M patients (Sanders 1 and 2) are prone to overcorrection.6,23 Optimal timing and tensioning of the cord at each spinal segment need to be studied further to minimize overcorrection. Our cohort of IM patients may be more skeletally M than others reported in the literature, which could account for our relatively low rates of overcorrection and revision surgery. Given these findings, we agree with others23,26 who concluded that tethering patients slightly after their growth acceleration phase26 may lead to more predictable successful outcomes, where much of the correction occurs intraoperatively.
Our compensatory TL/L curves corrected independent of skeletal maturity and by similar magnitudes as with PSF.5,9,20 We observed overcorrection of the uninstrumented TL/L compensatory curves in 2 Risser 2 patients, which has also previously reported.5 Normal kyphosis was maintained for the majority of patients regardless of skeletal maturity, which is congruent with prior literature.6,19,20 We also found that kyphosis below the mean for the cohorts increased at follow-up, whereas kyphosis above the mean decreased at follow-up as was desired. It is unclear to the surgeon what specific intraoperative maneuvers might account for those differences.
We report no difference in tether breakage with 2 suspected broken tethers per group. In 1 IM patient, cord breakage occurred at 39 months, coinciding with overcorrection by 2°. Cord breakage can be fortuitous if in an overcorrected segment might otherwise require a revision,19 which was not needed in our series. The M cohort only experienced 2 single cord breakages. We anticipate that tethers will fail in the future with higher incidence of failure in the more mobile sections of the spine. Longer-term follow-up is required to determine the impact of tether breakage on maintenance of correction. We now use a double cord for at least part of the construct to mitigate against cord breakage and loss of correction. Intraoperative translational and derotational maneuvers may also help achieve correction without overstressing and potentially damaging the tether.6
We found VBSQ of the curve apical vertebra was significantly increased in IM patients. We attribute this to enhanced bone remodeling of the vertebral body concave wall during the growth acceleration phase according to the Heuter-Volkmann law.2,10 One postmenarchal Risser 4 woman squared the apical wedge by 4° (Figure 8). While remaining growth could have accounted for this, bone remodeling by Wolff law may also contribute to an M patient’s vertebral remodeling. The unchanged UIV-LIV vertical height at YR2 in the M group suggests that the integrity of the tether-spinal column construct remains intact, perhaps through shared loading of a partially corrected spine, assisted by the tether.
Preoperative posteroanterior scoliosis radiograph of a mature group patient (Risser 4, proximal humerus ossification system 4 [PHOS 4]) with a 57° thoracic curve and 40° lumbar curve. First erect radiograph shows correction of major and compensatory curves. At 24 mo, the patient was Risser 5 (PHOS 5), and posteroanterior radiograph shows maintenance of clinically successful correction. Apical wedge angle was 12° at first erect and 8° at 2-y follow-up, calculating a VBSQ of 4°. Follow-up Cobb angles were measured between vertebra contained by the black bars, where white bars indicate preoperative angles. The yellow arrow indicates the apical vertebra, and yellow dots mark the vertebral body margins.
Limitations include our choice of skeletal maturity staging system. Sanders stage is most commonly reported in AVBT studies, but it requires additional hand radiographs, which were unavailable for all of our patients.25 Inaccuracies in Risser staging mostly exist between grades 3, 4, and 5 28; however, these patients were grouped as skeletally M in our study, lending confidence to our stratification. Furthermore, PHOS distributes excellently across the growth acceleration phase, and Risser stages were therefore confirmed with PHOS, thus permitting us to isolate a cohort of patients who had minimal remaining skeletal growth, which we defined as M.26,30,31 Last, VBSQ may be subject to inaccurate measurements secondary to spinal rotation. We therefore quantified VBSQ between FE and YR2 follow-up, as opposed to PR, thus bypassing the effect of operative curve derotation. These measurements are relatively small and may be subject to measurement variability.
Conclusion
AVBT is principally indicated for skeletally IM patients with primary thoracic curves between 35° and 60°. Nevertheless, the perception of achieving correction while avoiding PSF makes this technology appealing. Although, tethering more M patients is not widely accepted, our results reflect that these patients have satisfactory operative correction and appear to maintain correction at YR2. These patients may also benefit from low overcorrection risk without added mechanical complication rates. Further study relating to long-term outcomes and investigations into whether tissue remodeling occurs is warranted.
Footnotes
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests Dr Lonner reports personal fees, royalty fees, and research grant support from Zimmer Biomet for The Tether implant. Dr Lonner also reports personal fees, nonfinancial support and other from Depuy Synthes, personal fees and non-financial support from OrthoPediatrics, other from Paradigm Spine, non-financial support and other from Spine Search, and other from Setting Scoliosis Straight Foundation outside the submitted work.
Ethics Approval This work was approved by the Institutional Review Board at Mount Sinai Hospital.
Data Availability Statement Data will be maintained for this study for up to 7 years after publication as required by our institution’s review board.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2023 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.