ABSTRACT
Background: The optoelectronic camera source and data interpolation serve as the foundation for navigational integrity in the robotic-assisted surgical platform. The objective of the current systematic review serves to provide a basis for the numerical disparity that exists when comparing the intrinsic accuracy of optoelectronic cameras: accuracy observed in the laboratory setting versus accuracy in the clinical operative environment. It is postulated that there exists a greater number of connections in the optoelectronic kinematic chain when analyzing the clinical operative environment to the laboratory setting. This increase in data interpolation, coupled with intraoperative workflow challenges, reduces the degree of accuracy based on surgical application and to that observed in controlled musculoskeletal kinematic laboratory investigations.
Methods: Review of the PubMed and Cochrane Library research databases was performed. The exhaustive literature compilation obtained was then vetted to reduce redundancies and categorized into topics of intrinsic optoelectronic accuracy, registration accuracy, musculoskeletal kinematic platforms, and clinical operative platforms.
Results: A total of 147 references make up the basis for the current analysis. Regardless of application, the common denominators affecting overall optoelectronic accuracy are intrinsic accuracy, registration accuracy, and application accuracy. Intrinsic accuracy of optoelectronic tracking equaled or was less than 0.1 mm of translation and 0.1° of rotation per fiducial. Controlled laboratory platforms reported 0.1 to 0.5 mm of translation and 0.1°–1.0° of rotation per array. There is a huge falloff in clinical applications: accuracy in robotic-assisted spinal surgery reported 1.5 to 6.0 mm of translation and 1.5° to 5.0° of rotation when comparing planned to final implant position. Total Joint Robotics and da Vinci urologic robotics computed accuracy, as predicted, lies between these two extremes—1.02 mm for da Vinci and 2 mm for MAKO.
Conclusions: Navigational integrity and maintenance of fidelity of optoelectronic data is the cornerstone of robotic-assisted spinal surgery. Transitioning from controlled laboratory to clinical operative environments requires an increased number of steps in the optoelectronic kinematic chain and error potential. Diligence in planning, fiducial positioning, system registration, and intraoperative workflow have the potential to improve accuracy and decrease disparity between planned and final implant position. The key determining factors limiting navigation resolution accuracy are highlighted by this Cochrane research analysis.
INTRODUCTION
The fundamental technological challenge of navigation and robotic-assisted spinal surgery is that the virtual world needs to clearly represent the physical, real-time world. Among the multiple applications, variables, and equipment used in navigation and robotic-assisted spinal surgery, the optoelectronic camera source and data interpolation process serves as the foundation for navigational integrity and accuracy (or lack thereof) in the surgical platform. The spectrum of optoelectronic technology platforms is quite diverse, with use in sports performance activities such as speed skating and soccer,1–4 human ergonomics,5,6 clinical gait and motion analysis,7–12 musculoskeletal kinematics,13–20 and clinical operative procedures.21–44 To this end, the degree of accuracy and errors acceptable across optoelectronic motion measurement platforms differ considerably based on application.1 For example, fiducial arrays placed on anatomic pelvic landmarks of alpine skiers reported translation accuracy and errors of 8.37 ± 7.1 mm.45 Although considered adequate for the evaluation of positional or orientation-related differences in this athletic application, discrepancies of this magnitude would be unacceptable in the clinical operative setting. Technological advancements in the accuracy of optoelectronic marker–based systems over the past 20 years have facilitated the adoption and application of these platforms to the field of robotic-assisted spinal surgery.12,46,47 An ensuing plethora of journal publications have documented the safety, efficacy, and technical accuracy of navigation and robotic systems,21–24,30–32,35,48–55 operative surgical applications,25,33,56,57 and challenges of process workflow, learning curve, and training.28,30,31,50,51
Review of these publications reveals what could be defined as a significant discrepancy when comparing optoelectronic accuracy in the laboratory setting versus the clinical operative environment. An approximate 10-fold decrease in technical accuracy of final implant position (≤2 mm) in the clinical operative environment was observed compared with controlled musculoskeletal kinematic studies (≤0.2 mm) despite the use of nearly identical optoelectronic camera systems. Hence, the objective of the current systematic review serves to provide a basis for the numerical disparity that exists when comparing the intrinsic accuracy of optoelectronic cameras, accuracy observed in the laboratory setting, and accuracy in the clinical operative procedures. It is postulated that there exists a greater number of linkages in the optoelectronic kinematic chain when analyzing the clinical environment in the laboratory setting. This increase in data interpolation, coupled with intraoperative workflow challenges, reduces the degree of accuracy compared with that observed in controlled musculoskeletal kinematic laboratory investigations.
METHODS
A comprehensive systematic review of the PubMed and Cochrane Library research databases was performed. The time interval was unrestricted, but the majority of publications making up the basis of this analysis were from 2000 to the present. A combination of key search terms was stratified into the following: optoelectronic measurement systems, technical accuracy, experimental error, robotic-assisted surgery, spinal kinematics, and navigation. The search was limited to papers in the English language, indexed in peer-reviewed journals accessible through online searches, and all publications included required a bona fide PubMed identification (pmid) or digital object identifier (doi) citation. The exhaustive literature compilation obtained was then pooled in an EndNote file, vetted to reduce redundancies and categorized into topics pertinent to optoelectronic measurement system accuracy with specific reference to intrinsic accuracy, registration accuracy, musculoskeletal kinematic platforms, and clinical operative platforms. The primary tier for inclusion focused on publications that reported quantitative units of measure (microns, millimeters, and degrees) for intrinsic camera accuracy and tolerances, accuracy obtained in a controlled laboratory setting, and accuracy in the clinical operative setting. In various fields—spinal surgery, urologic surgery, and total joint replacement surgery—navigation and robotic accuracy are measured and compared with the Optotrak 3020 and Optotrak Certus systems (Northern Digital Instruments, Waterloo, Ontario, Canada). Several localized positions in a radiographic phantom are compared with the data acquired with the gold standard Optotrak systems. For example, individual da Vinci trials were registered to the mean Optotrak data using a rigid point-based registration method.
An extensive number of peer-reviewed journal publications have documented the use, efficacy, safety, and technical accuracy achieved with robotic-assisted spinal surgery. The focus in reviewing these publications was to highlight the technical accuracy observations and determine a basis for discrepancy between planned versus actual final implant position based on postoperative computed tomography (CT) images. In case studies where quantitative measurements were not reported, the Gertzbein and Robbins score (GRS) was adopted to calculate pedicle screw implant position.58 According to the GRS classification, screws centered within the pedicle are considered grade A, <2 mm from center is a grade B, a breach from 2 to 4 mm is grade C, a breach from 4 to 6 mm is grade D, and >6 mm is grade E. Grades of A and B (<2-mm pedicle breach) are considered clinically acceptable, and all other grades indicate malposition.
RESULTS
Three-Dimensional Cartesian Rigid Body Transformations
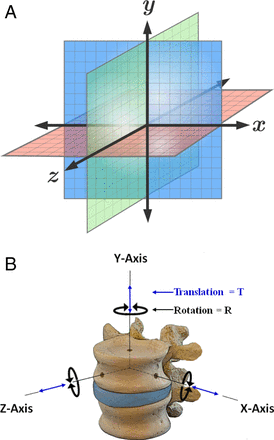
The reported optoelectronic measurements of accuracy, errors, and methods to quantify these in the laboratory setting or clinical operative environment are based on a fixed three-dimensional Cartesian coordinate system of rigid body transformation in millimeters of translation and degrees of rotation along three orthogonal axes: X, Y, and Z.59–63 This is in accordance to the axial (Y), sagittal (Z), and coronal (X) anatomic planes as defined by Panjabi's three-dimensional conceptual framework for spinal kinematics (Figure 1).64–66 From a nomenclature standpoint, accuracy is defined as a combination of trueness and precision according to the published International Organization for Standardization standard 5725-1.67 Trueness refers to the difference between measured value and true position, typically represented by the mean value of repeated measurements. Precision is a measure of repeatability, typically represented by the standard deviation of repeated measurements, and refers to random error and noise within the system. In addition to these standardizations, a useful key measure with regard to accuracy (trueness and precision) is the root mean square distance error (RMS) as given by ei being the three-dimensional distance error of measurement i and N the number of measurements:68

Cartesian Coordinate System and Conceptual Framework for Spinal Kinematics – Schematic representation of a fixed 3-dimensional Cartesian coordinate system for calculation of rigid body transformation in millimeters (mm) translation and degrees (deg) rotation along three orthogonal axes – X, Y and Z (A). This is in accordance to the axial (Y), sagittal (Z) and coronal (X) anatomic planes as defined by Panjabi's 3-dimensional conceptual framework for spinal kinematics (B).
Optoelectronic Measurement Systems
Image-guided surgery is based on the principle of integration and registration of the operative field to pre- or intraoperative data set (eg, CT or magnetic resonance imaging) via amalgamation of an optoelectronic imaging system with robotic platform.69 Although not necessarily involved in the execution of operative procedures, optoelectronic measurement systems are considered the gold standard in motion capture accuracy1,70 and provide three-dimensional visualization and guidance, improving task execution and targeting accuracy while functioning in a semiautonomous fashion.71 Hence, objective accuracy and error assessments of optoelectronic-robotic interventional platforms is essential. The Optotrak 3020 with a 24 infrared light-emitting diode (IRED) pen probe has a National Institute of Standards and Technology traceable accuracy of 0.1 mm for a single IRED over the work volume and a 0.25-mm accuracy when localizing a 24-IRED helical pen probe. Regardless of optoelectronic camera system, the fundamental triad of common denominators in assessing platform accuracy include (1) intrinsic accuracy of the source device, (2) registration and tracking accuracy, and (3) application accuracy. Prior to addressing the basis for application accuracy across laboratory versus clinical platforms, the intrinsic and registration accuracy and potential for error propagation are of primary consideration.
Intrinsic (Technical) Accuracy
The initial link in the optoelectronic kinematic chain of data transference resides in the intrinsic accuracy of the camera source. Of the multiple factors affecting downstream optoelectronic accuracy in musculoskeletal kinematic and clinical operative platforms, the intrinsic camera components are most controllable. Mechanical compliance of the system, loose interconnection mechanisms,69 variation in camera resolution, calibration, imperfect lenses, number of cameras, spatial orientation, noise, computer vision algorithms, and jitter all represent sources of intrinsic error in optoelectronic systems.47,69,72,73 Maletsky et al74 reported the relative accuracy position between two rigid bodies at 0.03 mm of translation and 0.04° of angulation, respectively. As a baseline statement of comparison, the optoelectronic systems used in clinical operative or controlled laboratory platforms report only marginal differences in accuracy. Further, the contribution of intrinsic errors is of miniscule value in comparison to error(s) propagation secondary to registration: targeting tracking and application in controlled experimental and clinical operative platforms.
Registration Accuracy and Target Tracking
A second key step in the optoelectronic kinematic chain and highest probable link(s) of error propagation is the registration process. This intraoperative process integrates correlation and mapping algorithms to register the physical patient to the virtual patient via the navigation system, optoelectronic source, fiducial arrays in the operative field, and coregistration of the patient intraoperative X-rays with the preoperative CT images. Accurate, close-to-ideal reference reproducibility and fidelity of the data set improves trueness and precision of subsequent intraoperative tracking. Multiple factors affect registration accuracy and target tracking, including optoelectronic camera source, passive versus active arrays, occlusions, distance between fiducial arrays and camera source, static versus dynamic array localization, and anatomic locations of the coordinate reference fiducials.1,35,44,55,68–81 For example, increasing the camera distance from 6 to 8 ft nearly triples the intrinsic registration error along the Z axis (maximum = 0.250 mm) for the Polaris passive fiducial array system. Hence, closer approximation of the optoelectronic camera source to the operative fiducial arrays (≤6 ft, or approximately 1800 mm) minimizes jitter and improves precision.82 In summary, propagation of computational measurement errors in the optoelectronic kinematic chain has a compounding effect for the following transitions: (1) measurement of the intrinsic image plane error secondary to errors within the optoelectronic system, (2) transitioning from image error to fiducial location error, and (3) transition from fiducial location error to tracking target error. The mathematical expressions for these computational transformations are beyond the scope of the current publication but are well documented by Fitzpatrick et al77,79 and Sielhorst et al.72 The margins of error secondary to intrinsic and registration accuracy in optoelectronics are more manageable compared with unpredictable factors related to application in the laboratory versus dynamic clinical intraoperative environments.
Application Accuracy: Basic Scientific Laboratory Platform
A plethora of publications have documented the biomechanical properties of the occipitocervical through lumbopelvic spine under controlled laboratory conditions using Panjabi's 3-dimensional conceptual framework for testing.17,64–66,83 In contrast to the challenges of the clinical operative environment, motion analysis of spinal implant and anatomic vertebral structures(s) in the controlled laboratory setting is performed using a 6-degree-of-freedom musculoskeletal simulator interfaced with an optoelectronic measurement system. The fundamental principles pertinent to maximizing optoelectronic accuracy include mounting the specimen to a rigid testing platform, affixing active or passive fiducial arrays directly to implants or anatomic structures using screw-bolt fixation, and creating rigid body configurations parallel to the camera source. To this end, a series of laboratory investigations using the NDI Certus and Vicon MX13 camera systems (Vicon Motion Systems Ltd, Oxford, UK) reported the peak limits of optoelectronic accuracy when evaluating kinematics of the osteoligamentous spine.13–19,84–90 Cunningham et al14 compared occipital plate versus intracranial anchors for reconstruction of the occipitocervical (O-C) junction. The reported differences (degrees) in axial rotation at the O-C junction based on optoelectronic measurements were 4.13 ± 2.05 (intact), 0.22 ± 0.13 (plate), and 0.30 ± 0.21 (anchor). Rotation of the plate and anchor with respect to the occiput in flexion-extension ranged from 0.06 ± 0.05 to 0.10 ± 0.08, respectively. Although not of clinical significance, the study quantified differences on the order of 0.1° between 2 methods of occipitocervical fixation.
In a complex kinematic study using a Vicon optoelectronic system, La Barbera et al18,19 investigated lumbar interbody cages with Ponte osteotomy versus pedicle subtraction osteotomy for severe sagittal imbalance. The peak accuracy of neutral zone measurements (degrees) across the intact L3–L5 segments demonstrated values of 0.7 (range 0.3–1.9) in flexion-extension, 1.0 (range 0.1–3.8) in lateral bending, and 0.2 (range 0.1–0.9) in axial rotation in flexion-extension. Factors of specimen stabilization, alignment, camera resolution, proximity to fiducials, planar visualization of the active arrays, and controlled motion application account for the high degree of accuracy reported in these studies. Laboratory workflow methods and conditions for experimental musculoskeletal kinematic studies are streamlined and optimized for maximizing optoelectronic accuracy. Factors of specimen stabilization, alignment, camera resolution, proximity to fiducials, planar visualization of the active arrays, and controlled motion application account for the high degree of accuracy reported in these studies. The collective effect of testing methodology and limited experimental coordinate transformations between data input/output reduces error propagation and maximizes optoelectronic accuracy (Figure 2, Table).91–97
Laboratory Platform for Optoelectronic Data Transference Process - Schematic illustration demonstrating the laboratory workflow and process for data transference utilizing optoelectronic tracking. The camera source visualizes the active fiducial arrays affixed to the vertebral elements and transfers the data directly to the user interface for computational analysis. The collective effect of testing methodology and limited experimental coordinate transformations between data input / output reduces error propagation and maximizes optoelectronic accuracy.
Robotic and navigation accuracy.
Application Accuracy: Clinical Operative Platform
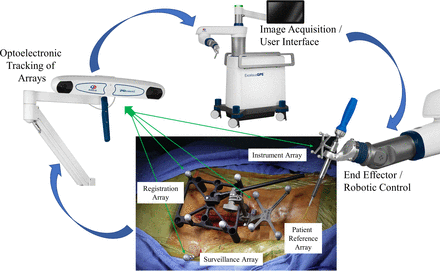
Transitioning from controlled laboratory conditions to the dynamic variability of a clinical operative environment presents a different set of application challenges for maintaining peak optoelectronic accuracy. Unique to robotic-assisted surgery and in contrast to the laboratory setting, the intraoperative process requires considerably more steps in the transference of optoelectronic kinematic data. This complex process flow integrates correlation and mapping algorithms to register the physical patient to the virtual patient via the navigation system, optoelectronic source, surveillance markers, patient reference markers, end effector instruments in the operative field, and patient CT images. Accurate, close-to-ideal reference reproducibility and maintenance of this data set is the primary intraoperative objective and challenge. Despite the use of nearly identical optoelectronic sources and fiducial arrays, a consistent disparity exists when comparing the reported technical accuracies in the laboratory setting versus clinical operative environment. An approximate 10-fold decrease in accuracy was observed when comparing the final implant position (≤2 mm) in the clinical operative environment with musculoskeletal kinematic studies (≤0.2 mm). An extensive number of peer-reviewed journal publications have documented the use, efficacy, safety, and technical accuracy achieved with robotic-assisted surgery.23,28,30,34–44,48,50–52,55,56,100–127 The focus in reviewing these publications across multiple robotic-assisted surgical platforms is to highlight the technical accuracy and discrepancy between planned versus actual final implant position (Table).
Based on the GRS system for transpedicular screw accuracy, Helm et al34 performed a comprehensive literature review on the technical accuracy of 1 622 pedicle screws implanted using a variety of image-guided surgery navigation systems. As reported, 11 830 were positioned Ideal according to preoperative plan (A), 395 screws within less than 2 mm of plan (B), 92 breached between 2 and 4 mm off center (C), and 55 were within 4 to 6 mm of the preoperative plan (Table). Grades of Ideal and A (<2 mm of plan) indicated “no perforations” and were considered acceptable in these anatomical segments according to Helm and coworkers. As a study limitation to the Helm et al publication, the paradigm of 3-dimensional accuracy as reported by Jiang et al128 is a more suitable and accurate description for pedicle screw placement versus accuracy based on single-plane imaging.
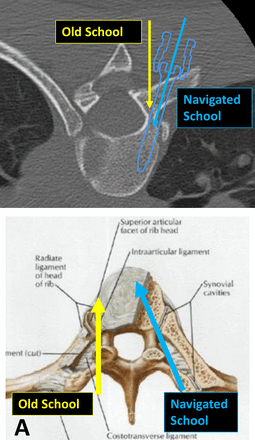
The time-honored tenet of pedicle screw deformity surgery, compared with European techniques for decades, was “probe rather than drill” the pedicle. This proved to be easier to learn and safer, as the surgeon could use a blunt probe and stay within the intramedullary canal of the pedicle and “increase accuracy.” The accuracy and safety of pedicle screw insertion was defined as reducing the number of pedicle screw breaches.129 With the advent of navigation and robotics, this definition has to be adapted to more contemporary goals (Figure 3). It is now possible to safely insert 4.5-mm-diameter pedicle screws into 3.0-mm thoracic pedicles by using navigated outside-in techniques, incorporating the entire costovertebral joint complex as a navigated target. Using a more tangential, outside-in converging trajectory, longer screws can be implanted compared with the previous “old-school,” nonnavigated techniques. Probing the pedicle and starting in the middle of the pedicle intramedullary canal allows the surgeon to concentrically enlarge the hole starting in the center. Unfortunately, using this old-school technique, the spinal canal can be breached in adolescent idiopathic scoliotic deformities, particularly on the convex side from T1 to T6, where the pedicles tend to be hypoplastic. With navigation, surgeons can optimize screw size in the upper thoracic pedicles: “drill to preserve the medial pedicle wall.” The precise, pinpoint location where the robot starts with orientation to the transverse process and superior articular process is unique to each pedicle. It is not in the center of the pedicle unless transpedicular depth and diameter are of sufficient dimension. The pedicle starting point, size, and optimal trajectory are planned based on a 3-dimensional virtual spine digitally constructed from a preoperative or early intraoperative CT scan. The starting point is unique to each pedicle screw and more tangential, especially in the cervical spine, compared with freehand techniques. The navigated trajectory is directed medial into the vertebral body and often more cranial—difficult to predict and accomplish freehand—which allows for improved purchase without violation of the medial wall. Using navigation and robotics in deformity procedures, the “new navigated school” allows for precise placement of costovertebral screws into the vertebral body. Therefore, the accuracy of robotic pedicle screw placement is based on the planned versus actual screw implant location, avoidance of a medial pedicle wall breach, and obtaining optimal fixation. The Gertzbein and Robbins quantitative scale of pedicle screw accuracy is not appropriate in the navigated school of robotic surgery, where intentional breach of the lateral pedicle and navigation of the pedicle screw into the costotransverse joint complex is intentional (Figure 4). This quantitative scale would result in a 100% intentional lateral breach despite increased fixation and correction of the scoliotic curve.
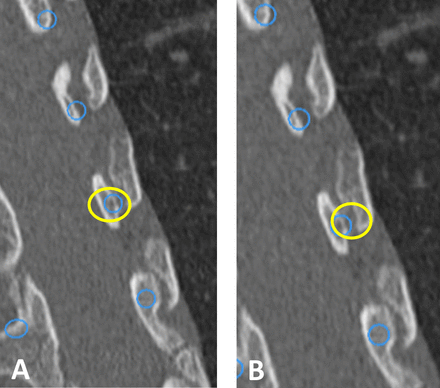
Computed Tomographic Images – Computed tomographic images demonstrating comparative pedicle screw insertion techniques of “Old School” versus “New School” methods. The Old School technique includes probing the center of the pedicle, intramedullary blunt pedicle finder, and concentrically expanding the pedicle to permit screw insertion (A). The New School navigated technique utilizes a more outside-in converging trajectory permitting preservation of the medial pedicle wall (B). Note the change in position of the circle defining region of insertion corridor.
Computed Tomographic and Schematic Images of Pedicle Screw Trajectory – The New Navigated School permits the surgeon to maximize pedicular fixation by incorporation of the entire costovertebral complex as showing the computed axial tomographic and schematic illustrations (A). As such, longer and larger diameter pedicle screws (5.5 mm versus 4.5 mm) can be inserted, while avoiding breach of the medial pedicle wall (B).
Continued.
In robotic-assisted total hip arthroplasty, Cozzi Lepri et al39 calculated differences between planned and actual measurements in leg length of 1.3 ± 1.5-mm displacement and target inclination of 0.3° to 0.7° angulation. As reported by Goia et al,43 for neurosurgical applications in deep brain stimulation, the difference between planned and actual lead implantations positions was on the order of 0.8 to 1.12 mm of displacement (Table). For urologic surgical procedures, the da Vinci robot has the most extensive track record, with several generations of improvements.130–132 As of December 2008, there were over 1000 da Vinci units sold, with well over 300 000 procedures performed. The most successful application of the robot is prostatectomy, with approximately 70% of all radical prostate removal procedures performed with the da Vinci in 2008 in the United States.132 A typical comparison of accuracy between the new da Vinci S model (1.25 mm) and the da Vinci Classic model (1.35 mm) are shown in the Table.98,99 As predicted, the intrinsic computed accuracy of the da Vinci urologic robot system is higher than robotic-assisted spinal applications (1.02 mm) but still not to the level of Optotrak (0.25 mm).98 Da Vinci systems are in their third generation—it is anticipated that the accuracy of spinal navigation and robotics will demonstrate similar improvements over the same time period with iterative improvements. Importantly, the da Vinci is a master-slave robot that does not incorporate real-time optoelectronic camera tracking. The current systematic review compares the da Vinci with studies that have been done with optoelectronic camera systems; however, this paradigm is not directly comparable to a real-time image-guided system that is used in spinal surgery. A direct comparison of accuracy between these 2 different robotic systems is challenging, as 2 different things are being compared (movement of a robotic arm in the case of the da Vinci and accuracy of screw placement in the case of a spine robot). While “accuracy” inferences can be made, a direct comparison between different robotic systems in different surgical subspecialties needs to take into account different definitions of “accuracy.”
The continuation in the adoption of navigation and robotic-assisted surgical procedures is ensured, as there is space for improvement in the accuracy based on Cunningham and Brooks's133 meta-analysis, which establishes the basic scientific accuracy: 0.1 mm of translation and 0.1° degree of rotation in optoelectronic laboratory conditions. Despite the use of nearly identical optoelectronic systems in the laboratory and clinical settings, the precision falls off in clinical spine applications to 3 to 4 mm translation and 2° to 3° of rotational accuracy. Robotic-assisted total joint replacement currently lies between these 2 extremes. Total joint robotics does not involve viscoelastic joints (3-joint complexes similar to the functional spinal unit) and does not involve a series of chain linkages between the navigated bone and skeletally anchored reference fiducials. Hence, the basis for the disparity and continuum of accuracy when transitioning from the controlled laboratory setting to the clinical operative environment is secondary to an increased number of steps in the optoelectronic kinematic chain and potential for error propagation in experimental coordinate transformations. Moreover, intraoperative challenges of array location, system registration, spinal flexibility, anatomic topography, and workflow affect navigational integrity and provide a basis for the disparity of optoelectronic accuracy in the clinical environment compared with the controlled laboratory setting. Collectively, these factors result in a continuum of optoelectronic accuracy with the greatest degree of accuracy observed in the laboratory setting and the least in the clinical operative spine environment (Table).
Basis for Disparity in Optoelectronic Accuracy
A key consideration pertaining to optoelectronic accuracy in the clinical environment compared with the laboratory setting is the dynamic nature of the operating room. The basis for disparity in accuracy when equating the laboratory versus clinical operative platforms is a result of the combined, cumulative errors secondary to the intraoperative workflow process, variability in anatomic morphology, and spinal flexibility. Of fundamental importance and the crux of the matter related to error propagation in navigation and robotic-assisted spinal surgery is the assumption that the workflow platform and patient's spine are rigid, and, as such, motion of any type is perceived as a rigid body transformation. Optoelectronic error reduction in the clinical flow requires stabilization of the camera source, rigid fixation of surveillance arrays in the iliac crest, stable attachment of patient reference and registration arrays to anatomic landmarks, and end effector instruments arrays that are inflexible. The end effector is the last link where the robotic enters the work space, and small rotations or translations in the array references can lead to large errors in instrument position. Although accurate, close-to-ideal reference reproducibility of these steps will reduce errors, the reality is that fixed arrays do move, leading to increased relative motions between arrays and subsequent error propagation and disparity between the physical, real-time world and the virtual world. Moreover, spatial errors can be further magnified due to geometrical distortion of preoperative images and tracking error of the surgical instruments.134 To register the physical patient to the virtual patient, Grunert et al44 proposed a series of transformation matrices, including fiducial-based paired-point transformation, surface contour matching, and hybrid transformation. The hybrid transformation process is most applicable to robotic-assisted spinal surgery, as it includes the methods of surface-based and pair-point–based methods with implanted fiducials. As such, tracing at least 3 anatomic landmarks with navigational confirmation serves to reduce error potential.
Several publications on optimizing clinical workflow process have been reported.31,49,53,134–138 Lieberman et al31 provides an excellent description of the step-by-step workflow process in robotic-assisted spinal surgery. The report provides a concise methodological approach to operative workflow while at the same time providing a collective basis for potential error(s) propagation in the clinical setting. The sequential description of process flow/error potentials includes preoperative and intraoperative registration, dislodgement of reference arrays, damaged or bent navigation tools139–141 and array occlusions (eg, distance and blood), skiving or tool deflection secondary to sloped anatomic topology or muscle retraction, and untracked patient movement during the spinal destabilization procedure. In addition to unintended motion or bending of fiducial arrays, the inherent differences in anatomic topology, bone mineral density, and flexibility of the patient's spine, both before and following destabilization and reconstruction procedures, cannot be overemphasized. The challenge is that the spine is often flexible—the drill and robotic arm may be properly located, but highly mobile, multisegmental spinal reconstructions with minimal deflection force lead to unintended rotation or translation of the operative vertebral elements, skiving, or tool deflection and affects precision rate during screw insertion.31,49,136,137,140,142,143 The basis for decreased technical accuracy in the clinical operative platforms is a result of combined, cumulative errors secondary to the intraoperative workflow process, the number of kinematic linkages, and variability in patient spinal morphology and flexibility (Figure 5).
Clinical Platform for Optoelectronic Data Transference Process – Schematic illustration demonstrating the operative clinical workflow and process for data transference utilizing optoelectronic tracking. Unique to robotic-assisted surgery and in difference to the laboratory setting, the intra-operative process requires considerably more steps in the transference of optoelectronic kinematic data. This complex workflow process integrates correlation and mapping algorithms to register the physical patient to the virtual patient via the navigation system, optoelectronic source, surveillance markers, patient reference markers, end effector instruments in the operative field, and patient CT images. Accurate, close-to-ideal reference reproducibility and maintenance of this dataset is the primary intra-operative objective and challenge.
DISCUSSION
In reviewing the intrinsic technical accuracy and registration accuracy, there exists a substantial burden of proof that the potential performance in optoelectronics is nearly identical between the two platforms—laboratory versus clinical operative—under static conditions. The downstream difference in optoelectronic technical accuracy and disparity between the 2 platforms is secondary to the dynamic factors unique to each. The laboratory workflow methods and array registration for experimental basic scientific studies are rigid and highly controlled with limited experimental coordinate transformations between data input/output, reducing error propagation and maximizing optoelectronic accuracy. Unique to robotic-assisted spinal surgery, the dynamic intraoperative process necessitates considerably more steps in the transference of optoelectronic kinematic data. The complex data flow process integrates correlation and mapping algorithms to register the physical patient to the virtual patient via the navigation system, optoelectronic source, surveillance markers, patient reference markers, end effector instruments, and patient CT images. Essentially, this is a comparison of technical accuracy between a rigid, highly controlled setting and a variable environment with multiple data input factors. The collective effect results in an increased potential for error propagation from experimental coordinate transformations, data processing, and optoelectronic kinematic linkages in the clinical setting.
There is significant potential for clinical improvements in spinal navigation and robotics. The current challenges as addressed in this Special Focus Issue are multifactorial:
The patient's thoracic spine moves under anesthesia with respirations as the chest cavity expands. This decreases the accuracy of placing pedicle screws on the upper convex side of adolescents with scoliotic curves.
An increased number of skeletally based reference fiducials are necessary and located closer to the target site.
Intervening spinal motion above and below spinal anchors, that is, Mazor L3–L4 motion when the Hover-T frame spans from the 2 pelvic Steinmann pins (PSIS) to the T12 spinous process (K-wire). The intercalated spinal segments that are bridged are free to move.
Need additional confirmatory skeletal reference fiducials to prevent skiving.
Learning through artificial intelligence would permit progressive incorporation of information as each pedicle screw is successfully inserted. The successive introduction of each pedicle screw should be merged into virtual information – leading to increased accuracy.
Clinical accuracy of robotics and navigation is not solely related to robotic sophistication. Fluoroscopic resolution used in registration and merging of the virtual data intraoperatively is often the gating item. Improvement in intraoperative 3-dimensional CT would increase accuracy and decrease operative time.
The future of spine robotics and navigation is essentially here—one only has to look at other fields. For example, otolaryngology has used electromagnetic fields for navigation rather than infrared camera capture of reflective skeletal fiducials. For intraoral resection of tumors, electromagnetic fields are advantageous, as they allow the advantage of non–line-of-sight navigation. This allows the surgeon to navigate around a corner or, in the case of otolaryngology, down the hypopharynx. There are 2 disadvantages of electromagnetic field navigation that have to be solved before this non–line-of-sight technology can be used in spine surgery. First, there cannot be ferromagnetic instruments in the field. Second, the distance (≤12 in) between the end effector and the field generator has to be reduced in order to have accurate navigation. The line-of-sight requirement for spinal navigation is a significant obstacle in current technology; it is invigorating that other surgical specialties are able to work through this obstacle.
There are several recent prospective randomized multi-institutional MIS trials that have relevance in the area of safety and accuracy of navigation and robotics. Good et al144 in their MIS ReFRESH study compared the complications and revision rates from Mazor robotics in 485 patients—374 robotic guidance arm and 111 patients in a fluoroscopy guidance arm from 9 sites. Fluoroscopic time per screw during instrumentation was 3.6 ± 3.9 s with Mazor compared with 17.8 ± 9 s with fluoro-guidance, indicating an 80% reduction in intraoperative radiation per screw (P < .001). During the first postoperative year, robotic guidance led to a 5.8 times lower risk of surgical complications and 11 times reduced risk of revision surgery. It is important to follow the progress of prospective randomized trials such as the MIS-ReFRESH study as more data are accumulated over longer time intervals. Staartjes et al145 pooled 3 randomized controlled trials, and Lieber et al28 are following 257 patients in a national inpatient sample with matched freehand controls. Gradually, implant accuracy should improve, complications should decrease, and revision rates should decrease as the early majority of fellowship-trained spinal surgeons gain more peer-reviewed and networked experience.
CONCLUSIONS
The fundamental technological challenge of navigation and robotic-assisted spinal surgery is that the virtual world needs to clearly represent the physical, real-time world. Navigational integrity and maintenance of fidelity in the transference of optoelectronic data is the cornerstone of robotic-assisted surgery. Transitioning from the controlled laboratory setting to the clinical operative environment requires an increased number of steps in the optoelectronic kinematic chain and potential for error propagation in experimental coordinate transformations. Moreover, intraoperative challenges of array location, system registration, spinal flexibility, anatomic topography, and workflow affect navigational integrity and provide a basis for the disparity of optoelectronic accuracy in the clinical environment compared with the controlled laboratory setting. A continuum of decreased accuracy is demonstrated when comparing the optoelectronic camera source itself with application in musculoskeletal platforms and, finally, the clinical operative environment. Diligence in the areas of preoperative planning, source camera and fiducial positioning, system registration, and intraoperative process workflow has the potential to improve accuracy and decrease disparity between planned and final implant position.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2021 ISASS
REFERENCES
- 1 .↵
- 2 .
- 3 .
- 4 .↵
- 5 .↵
- 6 .↵
- 7 .↵
- 8 .
- 9 .
- 10 .
- 11 .
- 12 .↵
- 13 .↵
- 14 .↵
- 15 .
- 16 .
- 17 .↵
- 18 .↵
- 19 .↵
- 20 .↵
- 21 .↵
- 22 .
- 23 .↵
- 24 .↵
- 25 .↵
- 26 .
- 27 .
- 28 .↵
- 29 .
- 30 .↵
- 31 .↵
- 32 .↵
- 33 .↵
- 34 .↵
- 35 .↵
- 36 .
- 37 .
- 38 .
- 39 .↵
- 40 .
- 41 .
- 42 .
- 43 .↵
- 44 .↵
- 45 .↵
- 46 .↵
- 47 .↵
- 48 .↵
- 49 .↵
- 50 .↵
- 51 .↵
- 52 .↵
- 53 .↵
- 54 .
- 55 .↵
- 56 .↵
- 57 .↵
- 58 .↵
- 59 .↵
- 60 .
- 61 .
- 62 .
- 63 .↵
- 64 .↵
- 65 .
- 66 .↵
- 67 .↵
- 68 .↵
- 69 .↵
- 70 .↵
- 71 .↵
- 72 .↵
- 73 .↵
- 74 .↵
- 75 .
- 76 .
- 77 .↵
- 78 .
- 79 .↵
- 80 .
- 81 .↵
- 82 .↵
- 83 .↵
- 84 .↵
- 85 .
- 86 .
- 87 .
- 88 .
- 89 .
- 90 .↵
- 91 .↵
- 92 .
- 93 .
- 94 .
- 95 .
- 96 .
- 97 .↵
- 98 .↵
- 99 .↵
- 100 .↵
- 101 .
- 102 .
- 103 .
- 104 .
- 105 .
- 106 .
- 107 .
- 108 .
- 109 .
- 110 .
- 111 .
- 112 .
- 113 .
- 114 .
- 115 .
- 116 .
- 117 .
- 118 .
- 119 .
- 120 .
- 121 .
- 122 .
- 123 .
- 124 .
- 125 .
- 126 .
- 127 .↵
- 128 .↵
- 129 .↵
- 130 .↵
- 131 .
- 132 .↵
- 133 .↵
- 134 .↵
- 135 .
- 136 .↵
- 137 .↵
- 138 .↵
- 139 .↵
- 140 .↵
- 141 .↵
- 142 .↵
- 143 .↵
- 144 .↵
- 145 .↵